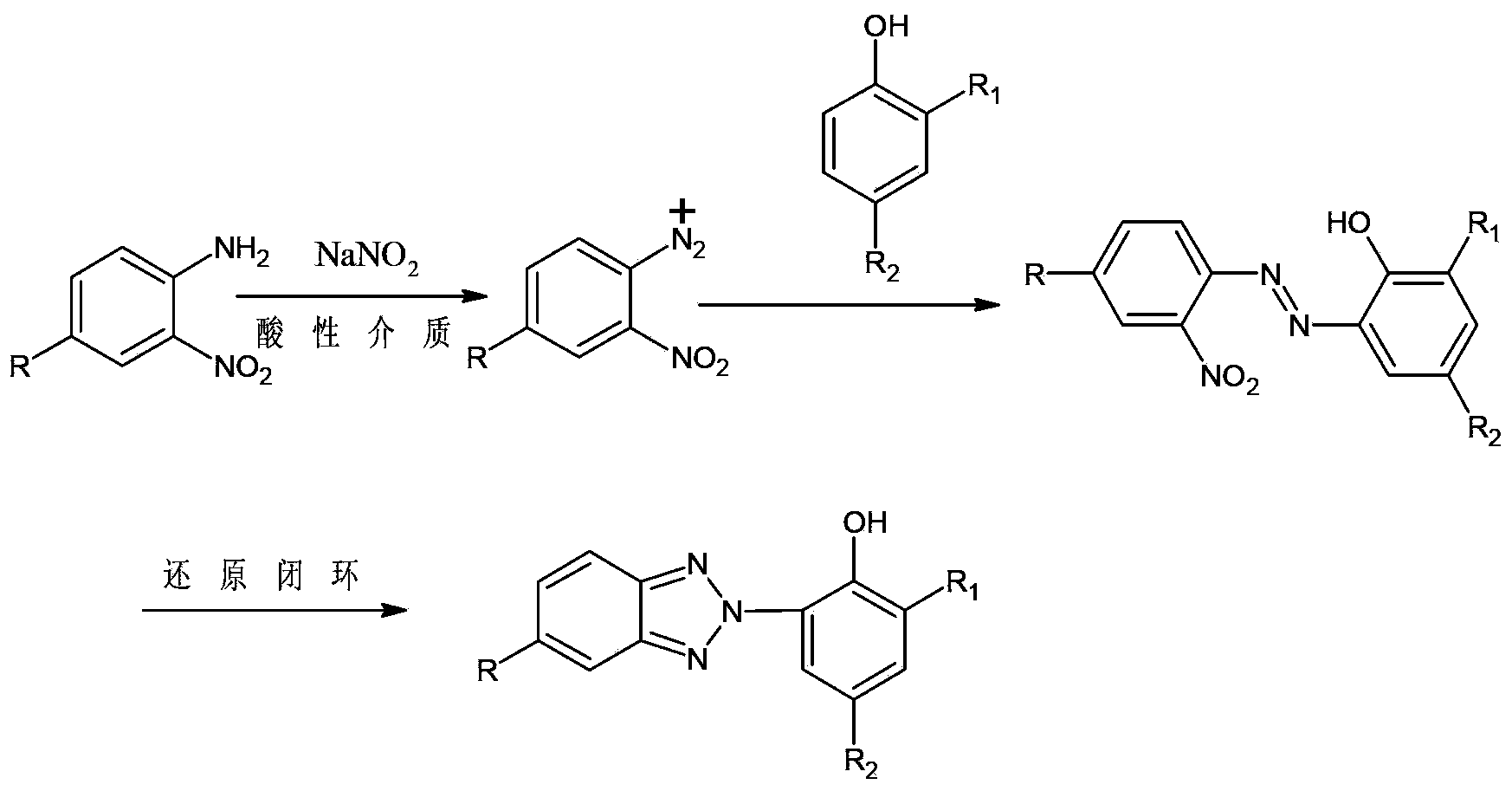
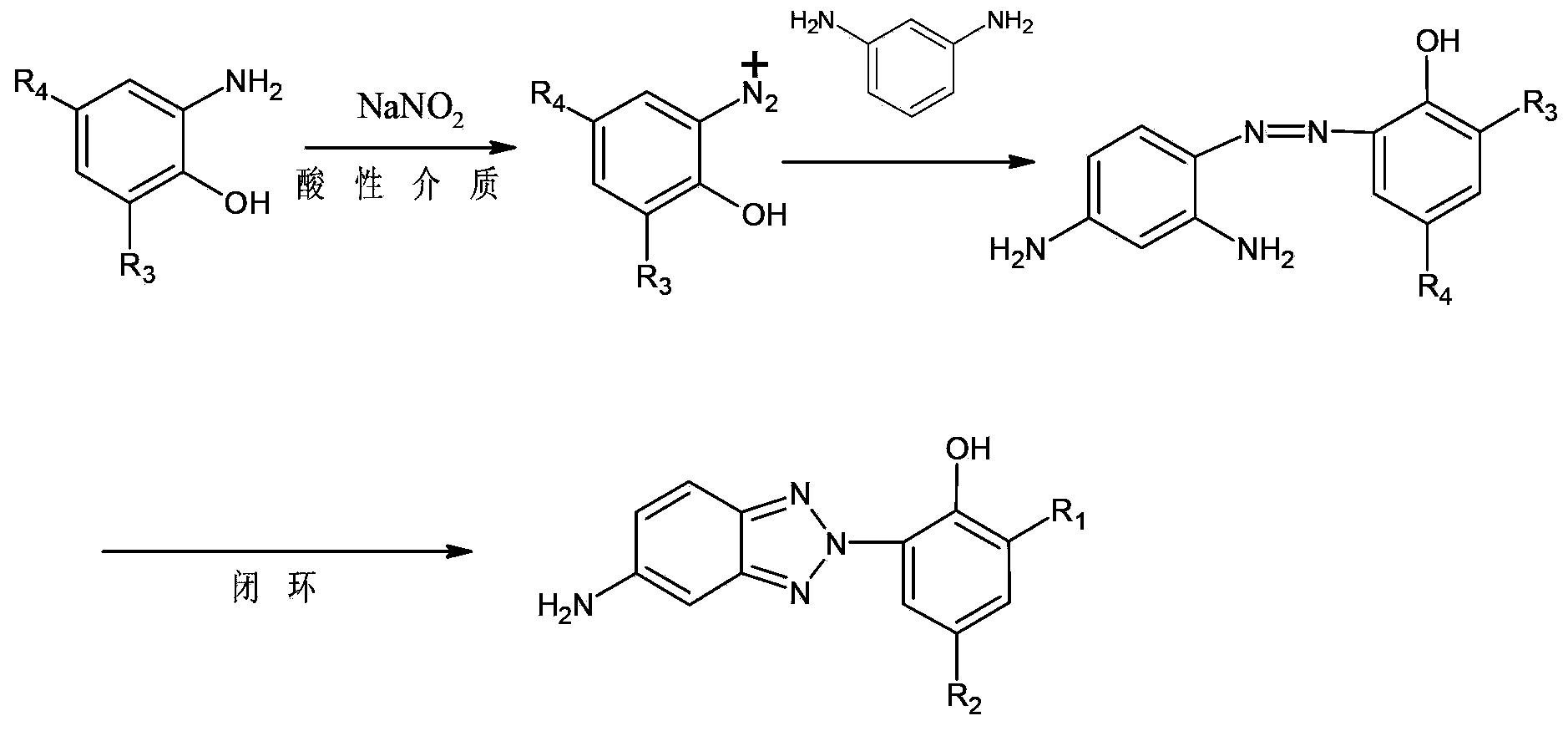
Synthetic method for benzotriazole ultraviolet absorbent UV-P
A UV-P, benzotriazole technology, applied in organic chemistry and other directions, can solve problems such as difficulty in large-scale production, poor product quality, and complicated post-processing, and achieve easy large-scale production, low cost, and convenient post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A preparation method for UV-P, comprising the steps of:
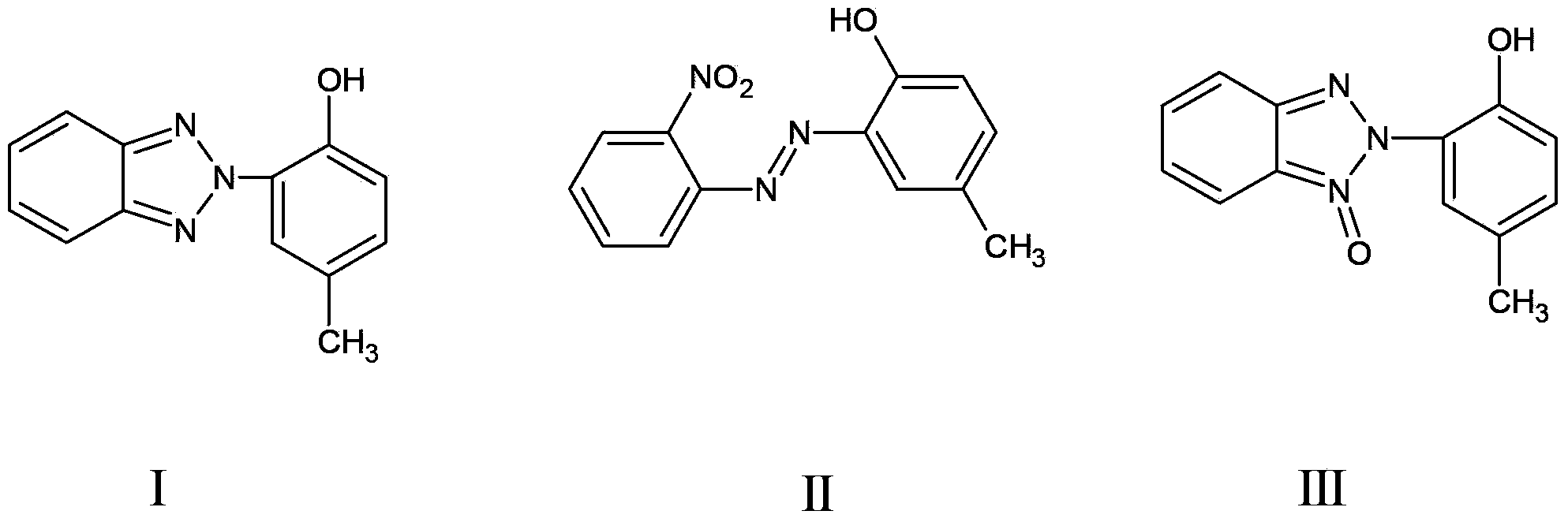
[0053] (1) Using the azo intermediate (II) as the raw material, the nitrogen oxide (III) can be obtained by reduction:
[0054] In a 500mL three-neck flask equipped with a stirrer and a reflux condenser, add 250g of dichloromethane, 25g of azo intermediates, and 15.17g of sodium sulfide in sequence, stir and raise the temperature to 40°C, heat and reflux at this temperature for 5h, and the reaction ends Afterwards, the reaction temperature was lowered to room temperature, and the reaction solution changed from dark red to earthy yellow. 200g of water was added into the reaction flask, stirred, extracted, the dichloromethane layer was dried, filtered, and the obtained filtrate was dissolved with nitrogen oxides (Ⅲ ) in dichloromethane, and the filtrate was kept for the next reduction.
[0055] (2) Using an organic solvent dissolved with nitrogen oxide (Ⅲ) as a raw material, catalytic reduction hydrogenation is us...
Embodiment 2
[0059] A preparation method for UV-P, comprising the steps of:
[0060] (1) Using the azo intermediate (II) as the raw material, the nitrogen oxide (III) can be obtained by reduction:
[0061] In a 500mL three-necked flask equipped with a stirrer and a reflux condenser, add 250g of 1,2-dichloroethane, 25g of azo intermediates, and 15.17g of sodium sulfide in sequence, stir and raise the temperature to 83°C, and heat to reflux at this temperature 4h, after the reaction was completed, the reaction temperature dropped to room temperature, the reaction solution changed from purple red to khaki, 200g of water was added into the reaction flask, stirred, extracted, the 1,2-dichloromethane layer was dried, filtered, and the obtained filtrate was For 1,2-dichloromethane dissolved with nitrogen oxide (III), the filtrate was saved for the next reduction.
[0062] (2) Using an organic solvent dissolved with nitrogen oxide (Ⅲ) as a raw material, catalytic reduction hydrogenation is used t...
Embodiment 3
[0066] A preparation method for UV-P, comprising the steps of:
[0067] (1) Using the azo intermediate (II) as the raw material, the nitrogen oxide (III) can be obtained by reduction:
[0068] In a 500mL three-necked flask equipped with a stirrer and a reflux condenser, add 114g of 1,2-dichloroethane, 0.1mol of azo intermediates, and 0.1mol of sodium sulfide in sequence, stir and raise the temperature to 88°C, and heat at this temperature Reflux for 2 hours. After the reaction, the reaction temperature dropped to room temperature, and the reaction solution changed from purple red to khaki. Add 100 g of water into the reaction flask, stir, extract, dry the 1,2-dichloromethane layer, and filter to obtain the filtrate It is 1,2-dichloromethane dissolved with nitrogen oxide (Ⅲ), and the filtrate is reserved for the next step of reduction.
[0069] (2) Using an organic solvent dissolved with nitrogen oxide (Ⅲ) as a raw material, catalytic reduction hydrogenation is used to prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com