Method for catalytically synthesizing mono-butyl itaconate at high selectivity
A technology for synthesizing monobutyl itaconic acid and selectivity is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc. The effect of short reaction time and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 13g of itaconic acid and 22.2g of n-butanol were weighed and heated and stirred in a three-necked flask, and the reaction temperature was controlled at 105°C. After stirring evenly, 0.26g of nanometer ZSM-5 clusters were added as a catalyst, and the reaction was carried out for 9 hours. Monobutyl itaconate was separated by centrifugation and crystallized at low temperature at 10°C as the initial product. During the reaction, it was quantified by GC-MS, and n-dodecane was used as internal standard.
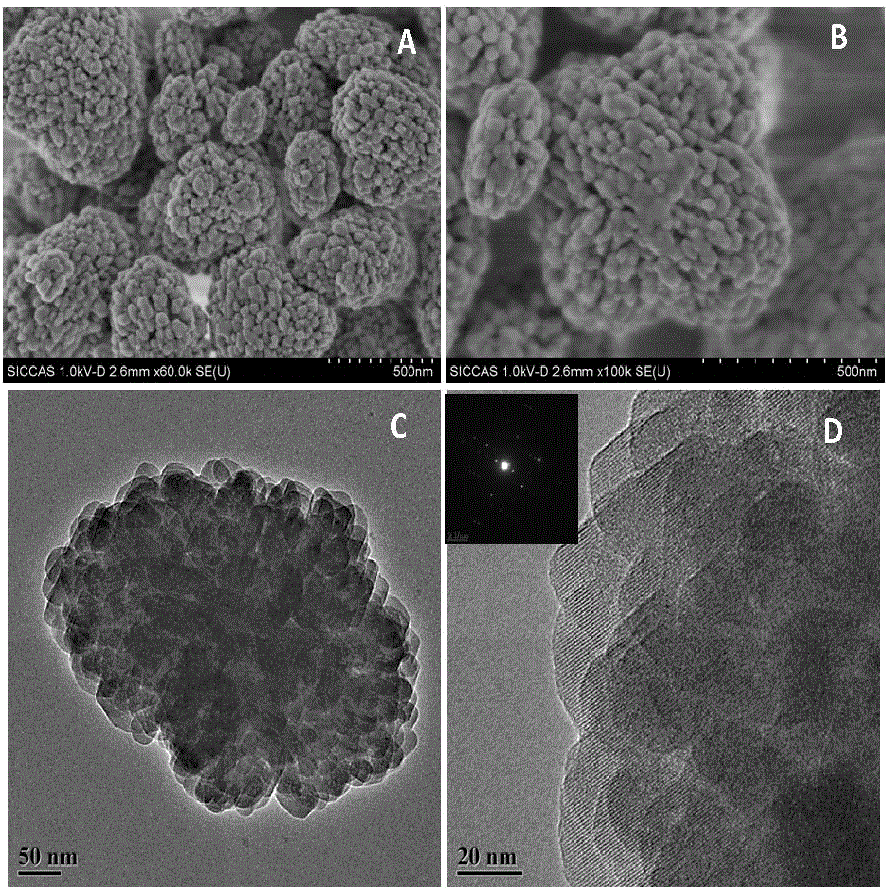
[0039] figure 1 It can be seen that the nanocrystalline clusters are small crystal grains of 30-80 nm in a certain orientation to form irregular spheres with a size of about 500 nm. The corresponding TEM images and electron diffraction patterns confirm that the arrangement of small crystal grains has a certain orientation, which is not a traditional polycrystalline structure.
[0040] see image 3 , in the present embodiment, the nanometer ZSM-5 cluster is used as a cataly...
Embodiment 2
[0042] The catalyst is large particle ZSM-5 zeolite, see figure 2 , the particle size is about 1500nm, and the specific surface area is 370m 2 g -1 , the pore volume is 0.15cm 3 g -1 . According to the method of Example 1, the corresponding catalytic performance test and cycle test were carried out.
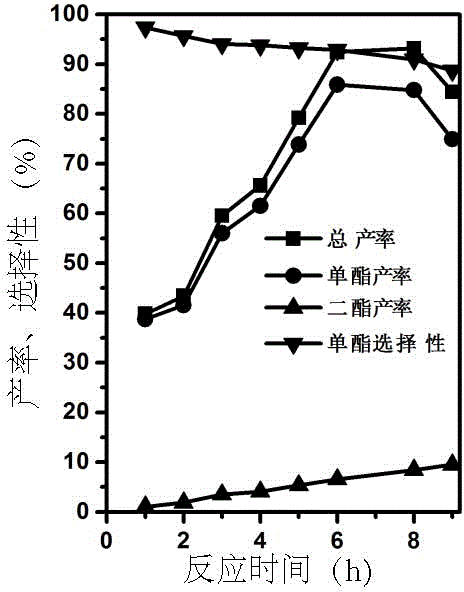
[0043] see Figure 4 , the traditional large zeolite in the butyl esterification reaction of itaconic acid, the generation rate of 6h monobutyl ester is 70%, the generation rate of dibutyl ester is 11%, and the selectivity of monobutyl ester is 86%. After the esterification of the carboxyl group at the β position of itaconic acid is completed, the diffusion distance from the pores is long, so part of the monoester continues to be esterified to form a diester, which reduces the reaction selectivity. On the other hand, the higher reactivity of nanocrystalline clusters was confirmed by the total ester yield of 81% for conventional zeolites relative to nanoclusters (93%).
Embodiment 3~5
[0045] The nano-ZSM-5 cluster catalyst in Example 1 was separated from the reaction system by centrifugation at 10000r / min for 10min, dried in an oven at 100°C for 2h, and then the mass ratio of the catalyst to itaconic acid was 2%, and the cycle test was carried out 3 times to verify The deactivation performance of the catalyst, the reaction temperature is about 105°C, and the catalytic reaction time is 6h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com