Novel thienopyridine compounds and use thereof in medicine
A compound and pyridine technology, applied in the field of new thienopyridine compounds and their medical applications, can solve problems such as low therapeutic efficacy, increased bleeding risk, and increased risk of massive bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
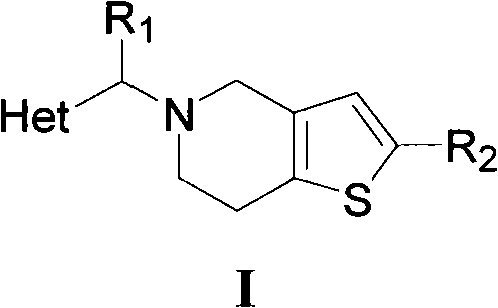
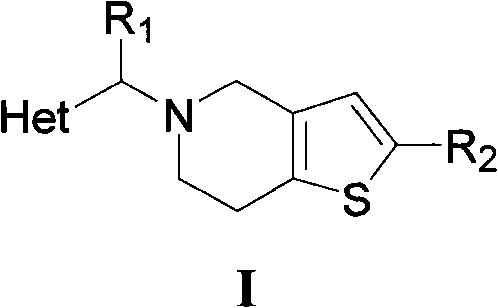
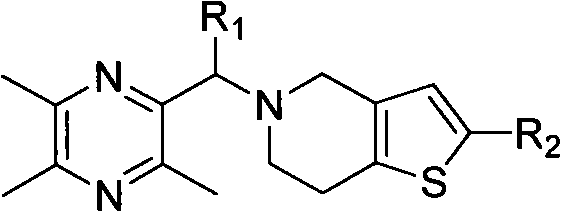
[0069] Example 1 5-(2-methylene-3,5,6-trimethylpyrazine)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride (compound I 1 )Synthesis
[0070] Dissolve 1.5g (11mmol) of 2,3,5,6-tetramethylpyrazine in 50mL of carbon tetrachloride, add 2.0g (11mmol) of NBS and a catalytic amount of benzoyl peroxide, reflux reaction under light, overnight React for 8-16 hours, monitor the reaction by TLC (ethyl acetate / petroleum ether=1 / 1), terminate the reaction after the raw materials disappear, cool to below 50°C, filter with suction, concentrate the filtrate under reduced pressure, and separate by column chromatography to obtain a white solid 2-Bromomethyl-3,5,6-trimethylpyrazine.
[0071] 1.75 g (10 mmol) of tetrahydrothienopyridine (THTP) hydrochloride was dissolved in 30 mL of dichloromethane, 1.2 equivalents of triethylamine (TEA) was added, and the reaction was stirred at 25° C. for one hour to obtain a mixed solution. Add 2.14g (10mmol) 2-bromomethyl-3,5,6-trimethylpyrazine, 2.0 eq...
Embodiment 2
[0072] Example 2 5-(2-methylenepyrazine)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride (compound I 2 )Synthesis
[0073] Dissolve 1.0g (11mmol) 2-methylpyrazine in 50mL carbon tetrachloride, add 2.0g (11mmol) NBS and a catalytic amount of benzoyl peroxide, reflux reaction under light, react overnight for 8-16 hours, TLC Monitor the reaction (ethyl acetate / petroleum ether=1 / 1), stop the reaction after the raw materials disappear, cool to below 50°C, filter with suction, concentrate the filtrate under reduced pressure, and separate by column chromatography to obtain light yellow liquid 2-bromomethylpyridine Zinc.
[0074] 1.75 g (10 mmol) of tetrahydrothienopyridine (THTP) hydrochloride was dissolved in 30 mL of dichloromethane, 1.2 equivalents of triethylamine (TEA) was added, and the reaction was stirred at 25° C. for one hour to obtain a mixed solution. Add 1.71g (10mmol) 2-bromomethylpyrazine, 2.0 equivalents of TEA and catalytic amount of NaI to the above mixed so...
Embodiment 3
[0075] Example 3 5-(2-methylenepyridine)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride (compound I 3 )Synthesis
[0076] Dissolve 1.0g (11mmol) 2-picoline in 50mL carbon tetrachloride, add 2.0g (11mmol) NBS and a catalytic amount of benzoyl peroxide, reflux reaction under light, react overnight for 8-16 hours, monitor by TLC Reaction (ethyl acetate / petroleum ether=1 / 1), the reaction was terminated after the raw materials disappeared, cooled to below 50°C, filtered with suction, concentrated the filtrate under reduced pressure, and separated by column chromatography to obtain 2-bromomethylpyridine as a yellow liquid.
[0077] 1.75 g (10 mmol) of tetrahydrothienopyridine (THTP) hydrochloride was dissolved in 30 mL of dichloromethane, 1.2 equivalents of triethylamine (TEA) was added, and the reaction was stirred at 25° C. for one hour to obtain a mixed solution. Add 1.70g (10mmol) bromomethylpyridine, 2.0 equivalents of triethylamine and catalytic amount of sodium iodide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com