Method for synthesizing manganese dioxide nanowire composite super absorbent resin
A manganese dioxide nanometer, super absorbent resin technology, applied in manganese oxide/manganese hydroxide and other directions, can solve the problems of poor salt resistance, slow water absorption, gel strength, dispersion elasticity and poor surface dryness, etc. Achieve the effect of improving salt tolerance, reducing production costs, and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
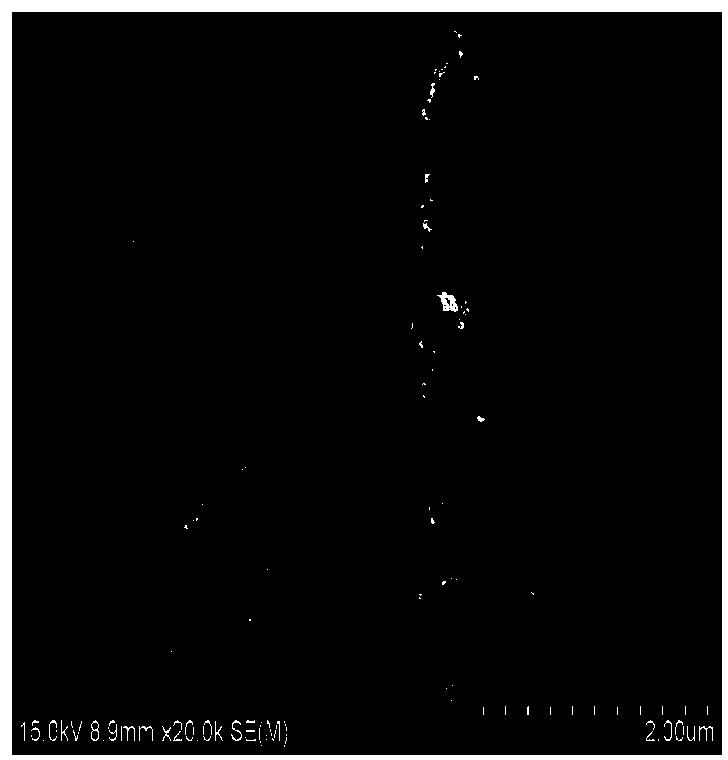
[0018] Take 4.1606g of potassium sulfate, 12.9076g of potassium persulfate, 4.1020g of manganese sulfate, add 100mL of deionized water, then put the mixed liquid into a stainless steel reactor lined with polytetrafluoroethylene, put the sealed reactor into Put it in an oven at 200°C for 24 hours. Then the generated solid is washed with distilled water at about 60°C for several times until the water-soluble impurities are removed, and dried at 80°C, the solid substance obtained is manganese dioxide nanowires, such as figure 1 As shown, the manganese dioxide nanowires were photographed by transmission electron microscopy figure 2 It can be seen that the diameter of the manganese dioxide nanowires is uniform.
[0019] Weigh 4.4445g of NaOH, dissolve it with 20mL of distilled water, place the beaker in an ice-water bath, slowly add 10mL of acrylic acid (AA) to the sodium hydroxide solution, adjust the neutralization degree of acrylic acid to 80%, and then add 6g of acrylamide (...
Embodiment 2
[0021] Take 4.1606g of potassium sulfate, 12.9076g of potassium persulfate, 4.1020g of manganese sulfate, add 100mL of deionized water, then put the mixed liquid into a stainless steel reactor lined with polytetrafluoroethylene, put the sealed reactor into Put it in an oven at 150°C for 24 hours. Then the resulting solid was washed with distilled water at about 60°C for several times until the water-soluble impurities were removed, and then dried at 80°C to obtain a solid substance that was manganese dioxide nanowires.
[0022] Weigh 1.556g of NaOH, dissolve it with 20mL of distilled water, place the beaker in an ice-water bath, slowly add 4mL of acrylic acid (AA) to the sodium hydroxide solution, adjust the neutralization degree of acrylic acid to 70%, and then add 6g of acrylamide (AM) and 2g of 2-acrylamide-2-methylpropanesulfonic acid (AMPS), stir evenly, then add 0.24g of manganese dioxide nanowires, 0.006g of cross-linking agent (N,N-methylene base bisacrylamide), 0.024...
Embodiment 3
[0024] 1 Preparation of manganese dioxide nanowires
[0025] Take 4.1606g of potassium sulfate, 12.9076g of potassium persulfate, 4.1020g of manganese sulfate, add 100mL of deionized water, then put the mixed liquid into a stainless steel reactor lined with polytetrafluoroethylene, put the sealed reactor into Put it in an oven at 180°C for 22 hours. Then the resulting solid was washed with distilled water at about 60°C for several times until the water-soluble impurities were removed, and then dried at 80°C to obtain a solid substance that was manganese dioxide nanowires.
[0026] Weigh 3.3334g of NaOH, dissolve it with 20mL of distilled water, place the beaker in an ice-water bath, slowly add 8mL of acrylic acid (AA) to the sodium hydroxide solution, adjust the neutralization degree of acrylic acid to 75%, and then add 6g of acrylamide (AM) and 2g of 2-acrylamide-2-methylpropanesulfonic acid (AMPS), stir evenly, then add 0.48g of manganese dioxide nanowires, 0.0112g of cross...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com