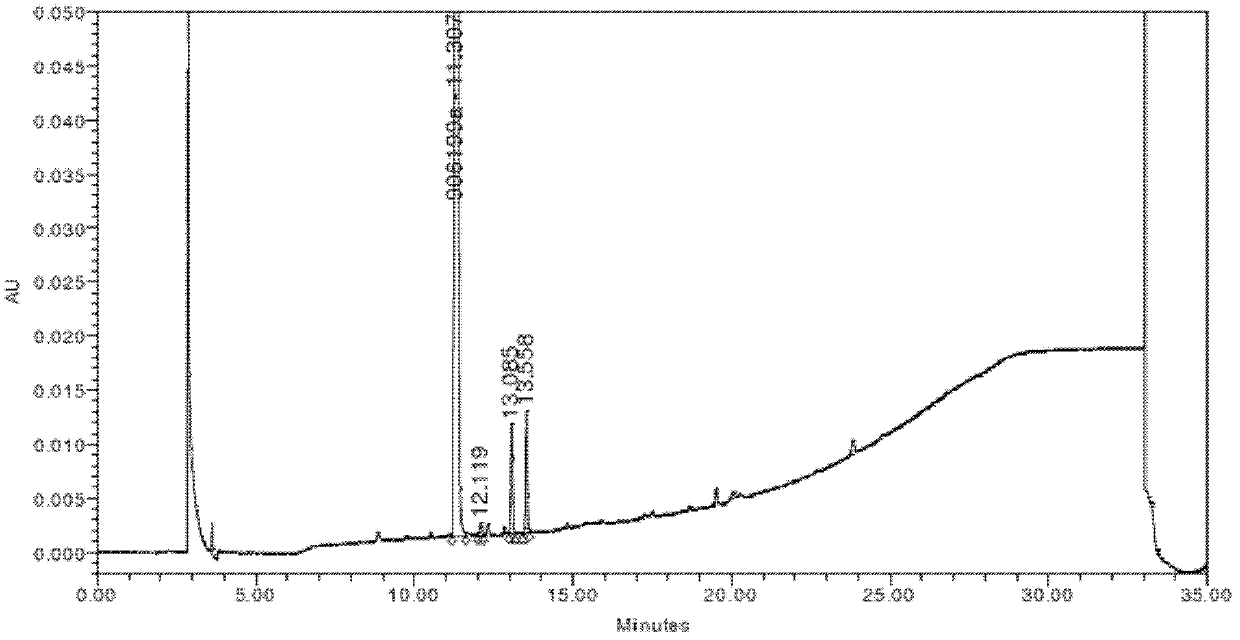
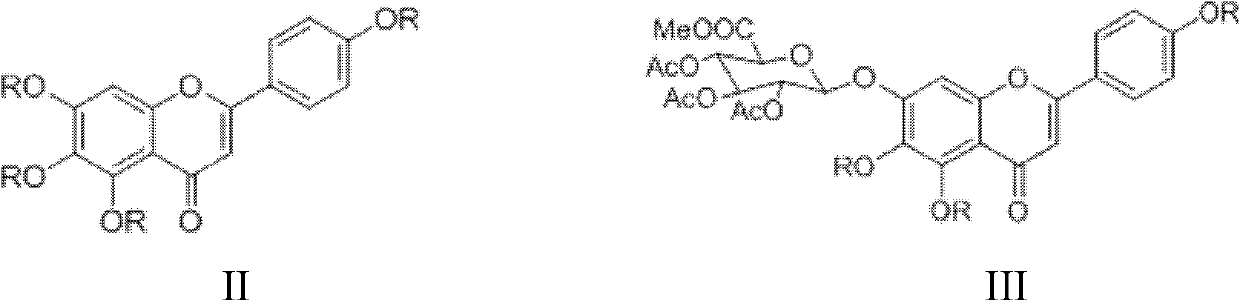
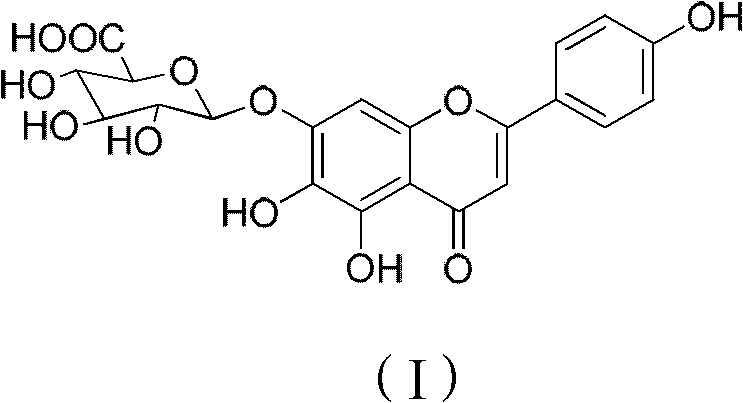
Method for preparing 5,6,4'-trihydroxy flavone-7-0-D-glucuronic acid
A technology of glucuronic acid and trihydroxyflavone, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of harsh reaction conditions, expensive reaction reagents, cumbersome process operations, etc., and achieve short synthetic routes , the product is easy to purify, and the effect of shortening the reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. Preparation of 5,6,7,4'-tetra-pivaloyloxyflavone
[0051] 20g (70mmol) of 5,6,7,4'-tetrahydroxyflavone was dissolved in 120mL of pyridine, and 173mL of pivaloyl chloride (d=0.976g / ML, 1.4mol) was slowly added dropwise at room temperature, and heated to 130°C for reflux reaction 6 After ~8 hours, TLC detected that the reaction was complete. When the reaction solution was cooled to about 70°C, 200 mL of ethyl acetate was added, and a large amount of white solid gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration gave 5,6,7,4'-tetrapivaloyloxyflavone as an off-white solid. After drying, it was recrystallized with ethanol to obtain 36.6 g of pure 5,6,7,4'-tetrapivaloyloxyflavone, a white solid, with a chromatographic purity of over 98.00% and a yield of 84%. 1H-NMR (400MHz, CDCl3), δ (ppm): 7.75 (2H, d, J = 8.6Hz), 7.13 (1H, s), 7.12 (2H, d, J = 8.6Hz), 6.48 (1H, s ), 1.59(9H, s), 1....
Embodiment 2
[0059] 1. Preparation of 5,6,7,4'-tetrabenzoyloxyflavone
[0060] Dissolve 10g (35mmol) of 5,6,7,4'-tetrahydroxyflavone in 70mL of pyridine, slowly add 81mL of benzoyl chloride (d=1.212, 700mmol) under stirring at room temperature, heat to 180°C and reflux for about 9 hours, TLC It was detected that the reaction was complete. When the reaction solution was cooled to about 70°C, 90 mL of ethyl acetate was added, and a large amount of white solids gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration afforded 5,6,7,4'-tetrabenzoyloxyflavone as an off-white solid. After drying, it was recrystallized with ethanol to obtain 19.9 g of pure 5,6,7,4'-tetrabenzoyloxyflavone, an off-white product, with a chromatographic purity of over 98.0% and a yield of 81%. 1H-NMR (400MHz, DMSO), δ (ppm): 8.56 (2H, d, J = 8.9Hz), 8.55 (2H, d, J = 8.9Hz), 8.54 (2H, d, J = 8.9Hz), 8.52 (2H, d, J = 8.9Hz), 8.10 (2H, d...
Embodiment 3
[0066] 1. Preparation of 5,6,7,4'-tetraacetoxyflavone
[0067] Dissolve 10g (35mmol) of 5,6,7,4'-tetrahydroxyflavone in 50mL of pyridine, slowly add 30mL of acetic anhydride (d=1.08, 400mmol) dropwise at room temperature, heat to 140°C and reflux for 4-5 hours, TLC The detection reaction is complete. When the reaction solution was cooled to about 70°C, 90 mL of ethyl acetate was added, and a large amount of white solid gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration afforded 5,6,7,4'-tetraacetoxyflavone as an off-white solid. After drying, it was recrystallized with ethanol to obtain 13.7 g of pure off-white 5,6,7,4'-tetraacetoxyflavone, with a chromatographic purity of over 98.00% and a yield of 86%. 1H-NMR (400MHz, CDCl3), δ (ppm): 7.64 (2H, d, J = 8.7Hz), 7.03 (1H, s), 7.02 (2H, d, J = 8.6Hz), 6.39 (1H, s ), 2.21(3H, s), 2.14(3H, s), 2.12(3H, s), 2.11(3H, s).
[0068] 2. Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com