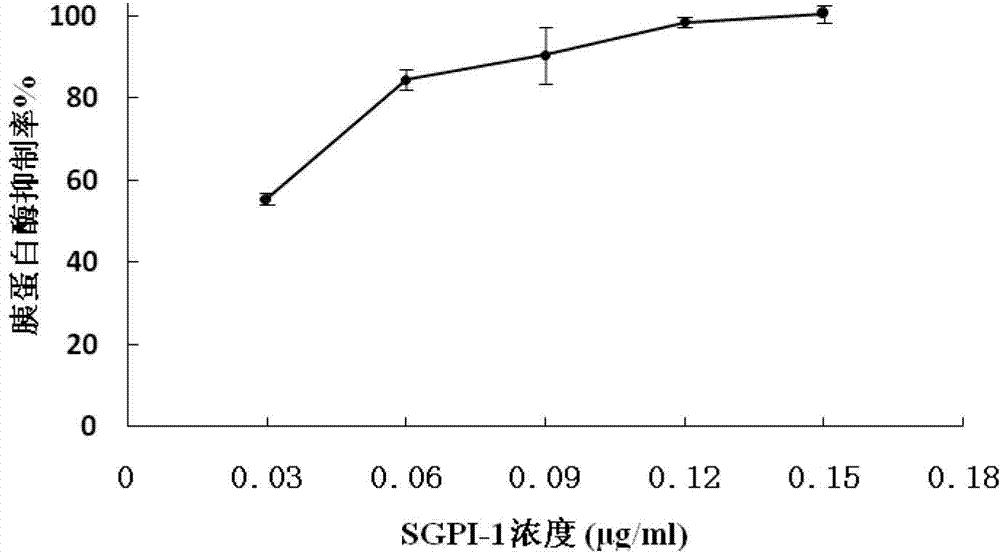
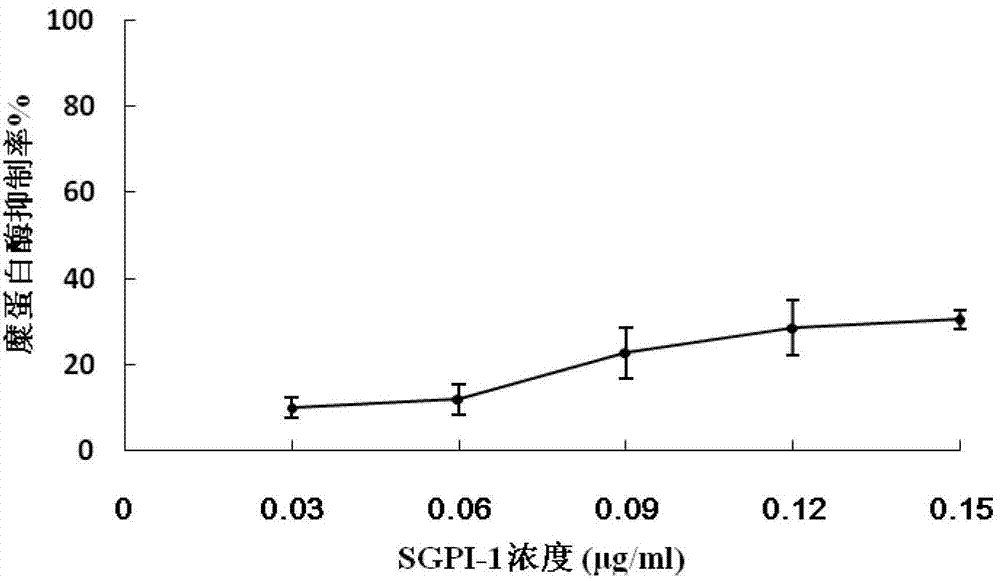
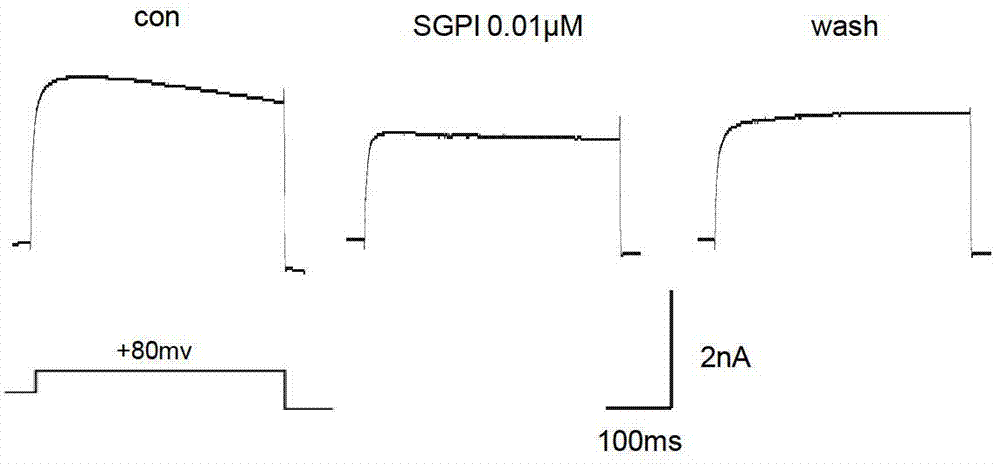
Sea anemone Kunitz-type protease inhibitor and application thereof
A protease inhibitor, trypsin inhibition technology, applied in the direction of protease inhibitors, applications, peptide/protein components, etc., can solve problems such as limited research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Separation and purification of sea anemone protease inhibitor SGPI-1
[0024] Take 5g of the tentacle tissue of the giant row finger sea anemone, cut it into pieces, add 20ml of cold deionized water to homogenate, and centrifuge at 15000rpm for 20 minutes to remove the residue. Filtrate through a 0.45 μm filter to obtain the crude poison of sea anemone toxin and store it at -75°C. Take 1ml of the crude poisonous sample and put it on the Hitrap Capto S cation exchange column, which is pre-balanced with 0.05mol / L ammonium acetate buffer solution (pH5.5). After sample loading, use 10%, 30% and 100% 1mol / L sodium chloride buffer solution (containing 0.05mol / L ammonium acetate, pH5.5) for gradient elution, each gradient elution time is 5min, and the flow rate is 1ml / min. 100% of the eluted fractions were pooled and lyophilized. The lyophilized sample was reconstituted with 0.15mol / L ammonium acetate buffer (pH5.5), and 2ml was passed through a HiLoad16 / 60Sup...
Embodiment 2
[0025] Example 2: Total RNA Extraction and cDNA Synthesis of Tentacles of Carpet Sea Anemone
[0026] RNase-free utensils were prepared, and the extraction of total RNA from tentacles of the carpet sea anemone was performed according to the instructions of RNAiso Plus from TaKaRa Company; the cDNA synthesis was operated according to the instructions of Fermentas RevertAid First Strand cDNA Synthesis Kit. Two bands of 18S and 28S can be seen in the extracted total RNA by electrophoresis, indicating that the integrity of the total RNA is good.
Embodiment 3
[0027] Embodiment 3: Cloning, sequence determination and analysis of SGPI-1 gene
[0028] According to the results of sequencing with the N-terminal of SGPI-1, degenerate primers were designed, and the primer sequences were as follows:
[0029] TGCTCTGAACCAAARGTNGTTGG (SEQ ID NO: 3).
[0030] Using the cDNA obtained in Example 2 as a template, the 3'-RACE kit (TaKaRa) was used to clone the SGPI-1 gene. Agarose electrophoresis detection, there is a specific amplification band around 200bp. This band was recovered and sent to Shanghai Yingjun Biotechnology Co., Ltd. (Invitrogen) for nucleic acid sequence determination. The obtained sequence was subjected to blast homology analysis. New toxin protein.
[0031] The DNA sequence is as follows:
[0032] TCTGGAATTTGCTCTGAACCAAAAGTTGTTGGCCGGTGTCGAGGAAGTTTTTCCAAGATTCTACTTCGATTCAGAGACGGGAGAGTGCAAACCCTTCATCTACGGTGGATGCGGGGGAAATGGGAATAACTTTGAGACCCTGCATCAATGCCGAGCTATATGCAGGGCG TAA (SEQ ID NO:1, the underlined part is not translated) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com