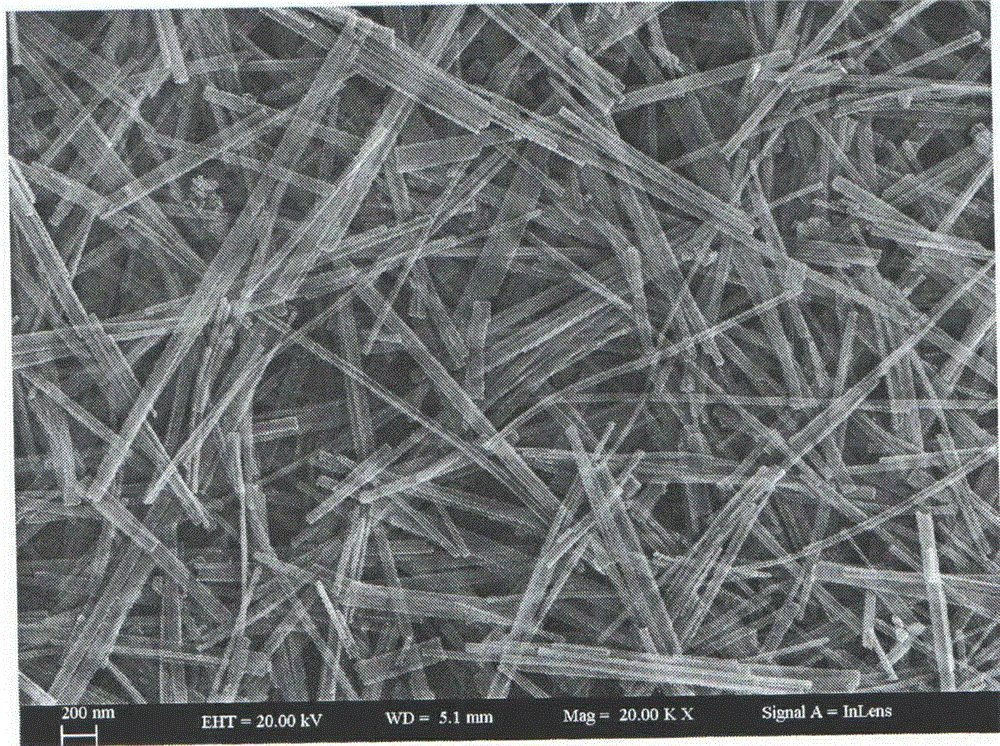
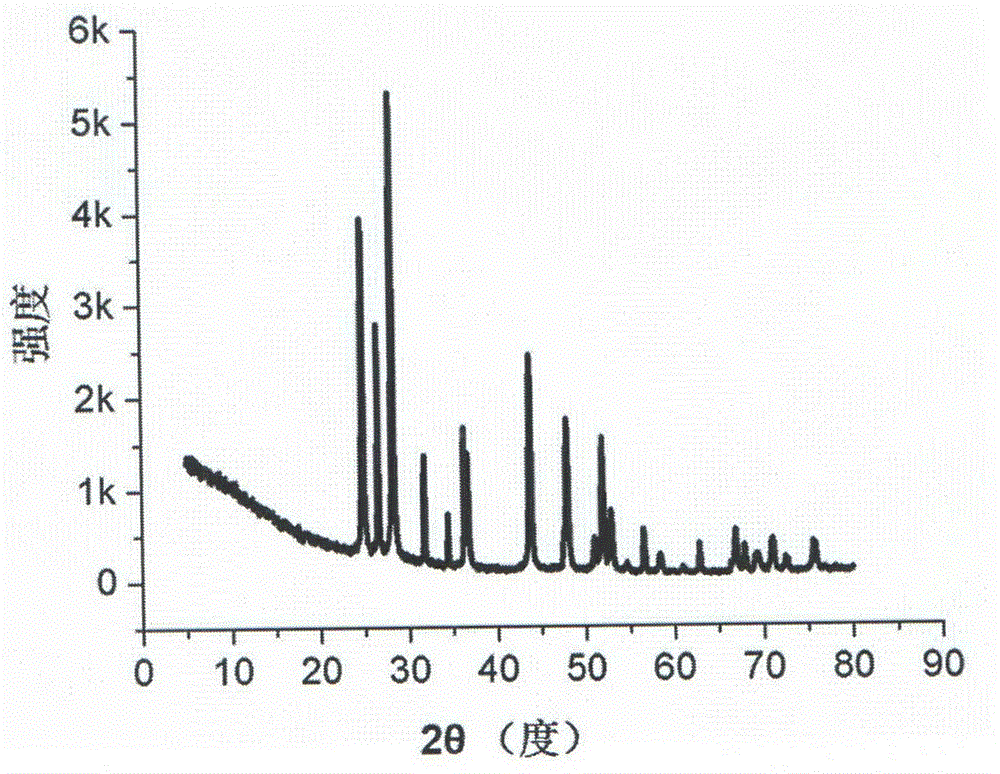
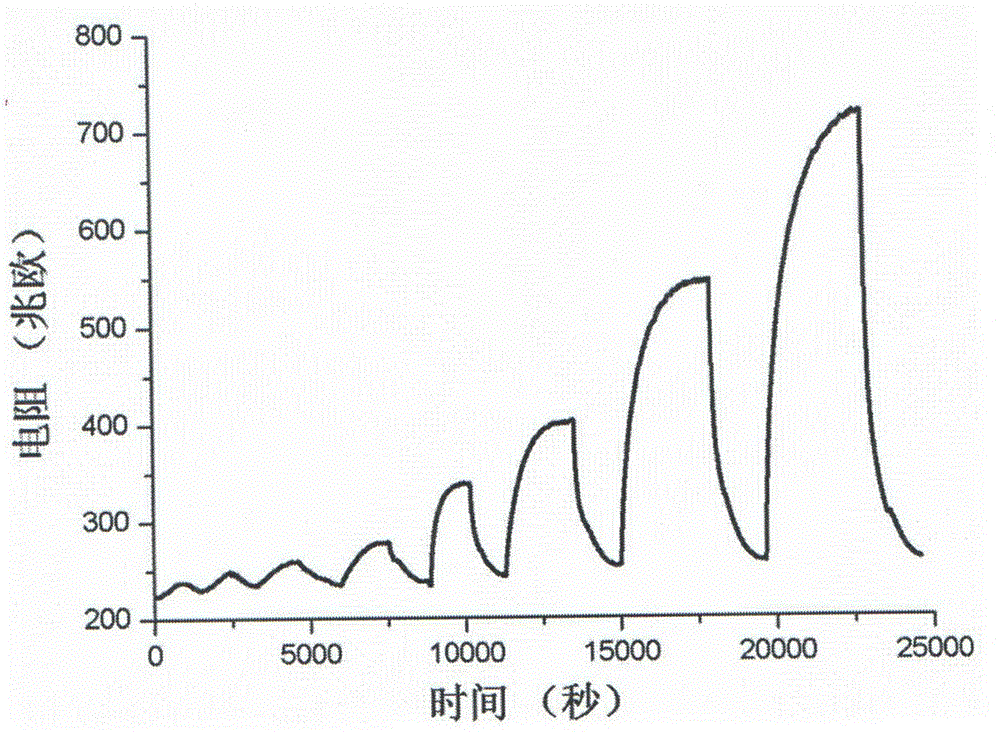
Cadmium sulfide/zinc oxide nuclear shell nanowire nitrogen dioxide sensing material and preparation method thereof
A core-shell nano-sensing material technology, applied in zinc oxide/zinc hydroxide, cadmium sulfide, nanotechnology, etc., can solve the problems of obvious photoelectric response and narrow CdS band gap.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] a. The raw materials cadmium nitrate and thiourea are dissolved in the solvent ethylenediamine at a molar ratio of 1:2. After the raw materials are dissolved, the solution is transferred to a Teflon hydrothermal kettle, reacted at a temperature of 160°C for 12 h, and cooled to room temperature;
[0022] b. Filter the resulting yellow precipitate, wash it with absolute ethanol and deionized water until no ethylenediamine remains, and dry it in vacuum at a temperature of 25°C for 4 hours to obtain a dry product;
[0023] c. Take 0.1 g of the dried product, add it to 60 mL of deionized water, ultrasonicate for 30 min to make it fully dispersed, then add 0.026 g of zinc acetate and 0.025 g of hexamethylenetetramine, stir evenly, and transfer to Teflon In a dragon water hot kettle, react at a temperature of 70°C for 6h, and cool to room temperature;
[0024] d. Filter the resulting yellow precipitate, wash it with deionized water until no hexamethylenetetramine remains, and...
Embodiment 2
[0027] a. The raw materials cadmium nitrate and thiourea are dissolved in the solvent ethylenediamine at a molar ratio of 1:4. After the raw materials are dissolved, the solution is transferred to a Teflon hydrothermal kettle, reacted at a temperature of 200°C for 72 hours, and cooled to room temperature;
[0028] b. Filter the resulting yellow precipitate, wash it with absolute ethanol and deionized water until no ethylenediamine remains, and dry it in vacuum at 85°C for 4 hours to obtain a dried product;
[0029] c. Take 1.0 g of the dried product, add it to 60 mL of deionized water, ultrasonicate for 30 min to make it fully dispersed, then add 0.52 g of zinc acetate and 0.5 g of hexamethylenetetramine, stir evenly, and transfer to Teflon In a dragon water hot kettle, react at a temperature of 150°C for 15 h, and cool to room temperature;
[0030] d. Filter the resulting yellow precipitate, wash it with deionized water until no hexamethylenetetramine remains, and dry it in ...
Embodiment 3
[0033] a. The raw materials cadmium nitrate and thiourea are dissolved in the solvent ethylenediamine at a molar ratio of 1:3. After the raw materials are dissolved, the solution is transferred to a Teflon hydrothermal kettle, reacted at a temperature of 180°C for 48 hours, and cooled to room temperature;
[0034] b. Filter the resulting yellow precipitate, wash it with absolute ethanol and deionized water until no ethylenediamine remains, and dry it in vacuum at 65°C for 4 hours to obtain a dry product;
[0035] c. Take 0.35 g of the dried product, add it to 60 mL of deionized water, ultrasonicate for 30 min to make it fully dispersed, then add 0.052 g of zinc acetate and 0.05 g of hexamethylenetetramine, stir well, and transfer to Teflon In a dragon water hot kettle, react at a temperature of 120°C for 12 h, and cool to room temperature;
[0036] d. Filter the resulting yellow precipitate, wash it with deionized water until no hexamethylenetetramine remains, and dry it in v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com