Preparation method of recessed alpha-phase ferric oxide cube
A ferric oxide, cube technology, applied in the direction of iron oxide, iron oxide/iron hydroxide, etc., to achieve the effect of uniform distribution, uniform particle size and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Weigh 0.8080g (about 2mmol) of iron nitrate (Fe(NO 3 ) 3 9H 2 O), it was dissolved in 10 mL of deionized water, and 0.199 g (about 1 mmol) of copper acetate (Cu(CH 3 COO) 2 ·H 2 O), stir well to dissolve. Add 0.15 g of polyvinylpyrrolidone (average molecular weight: 40,000) to the above solution, and dissolve it by ultrasonication.
[0019] (2) Add 10 mL of ammonia water with a mass fraction of 25% to the solution obtained in step (1), and mix thoroughly after ultrasonication for 3 minutes.
[0020] (3) Add 2mL of formamide (analytical grade) to the solution obtained in step (2), then transfer to a 50mL Teflon-lined stainless steel reaction kettle, seal and heat up to 160°C for 12h .
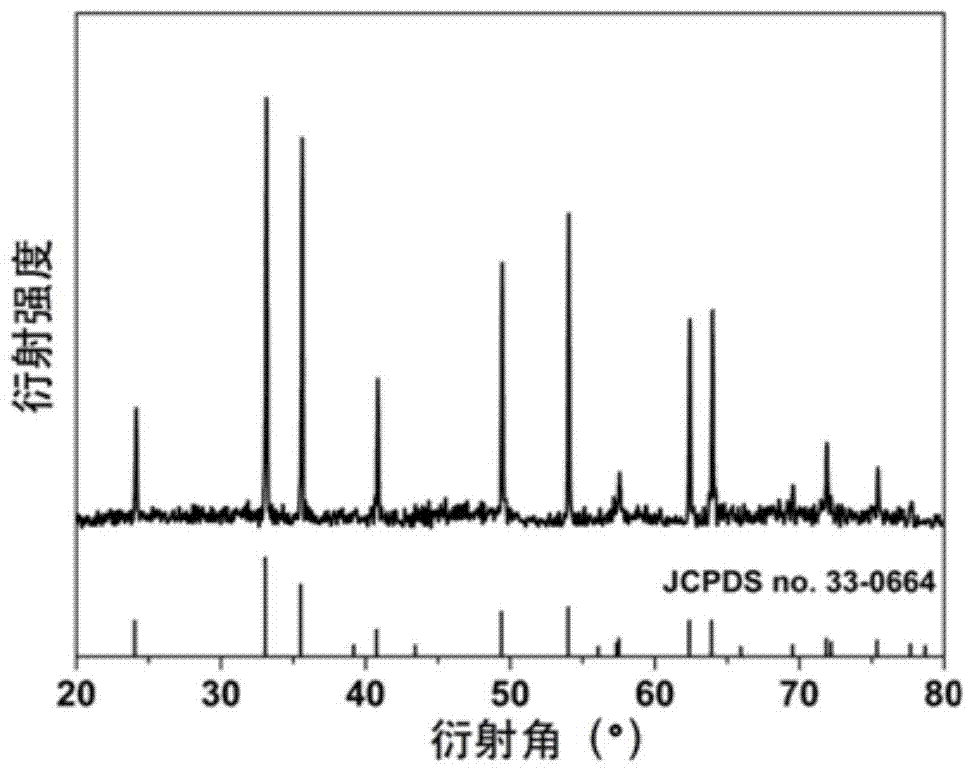
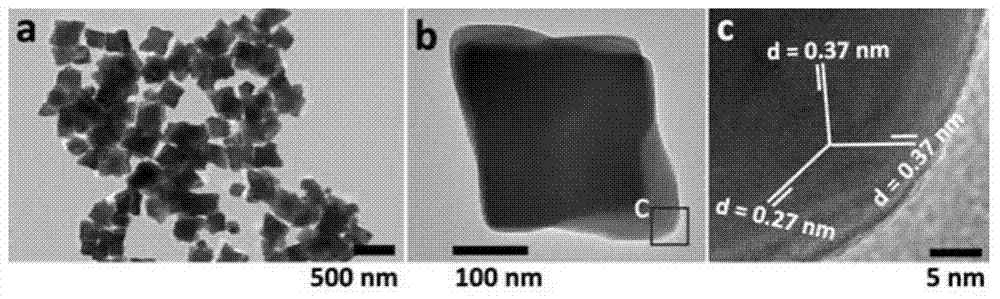
[0021] (4) Cool down to room temperature naturally after the reaction is complete, centrifuge the sediment at the bottom of the kettle, wash with absolute ethanol and deionized water for 3 times, and dry the obtained product at 60°C for 12 hours to obtain α-phase ferric oxide ...
Embodiment 2
[0027] (1) Same as embodiment 1.
[0028] (2) Same as embodiment 1.
[0029] (3) Add 1mL of formamide (analytical grade) to the solution obtained in step (2), then transfer it to a 50mL Teflon-lined stainless steel reactor, seal it and raise the temperature to 150°C Keep warm for 36 hours.
[0030] (4) Same as embodiment 1.
Embodiment 3
[0032] (1) Weigh 0.54g (about 2mmol) of ferric chloride (FeCl 3 ·6H 2 O), it was dissolved in 20 mL of deionized water, and 0.5 g (about 2 mmol) of copper sulfate (CuSO 4 ·5H 2 O), stir well to dissolve. 0.1 g of polyvinylpyrrolidone (average molecular weight: 55,000) was added to the above solution, and dissolved by ultrasonication.
[0033] (2) Add 20mL of ammonia water with a mass fraction of 25% to the solution obtained in step (1), and mix thoroughly after ultrasonication for 3 minutes.
[0034] (3) Add 2 mL of formamide (analytical grade) to the solution obtained in step (2), then transfer it to a 100 mL Teflon-lined stainless steel reaction kettle, seal it and raise the temperature to 160 °C Keep warm for 24 hours.
[0035] (4) Same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com