Preparation method of hydrazide compound
A compound and hydrazide technology, applied in the field of preparation of hydrazide compounds, can solve the problems of explosion, environmental pollution, strong reducibility, etc., and achieve the effects of small safety risk, wide application range and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
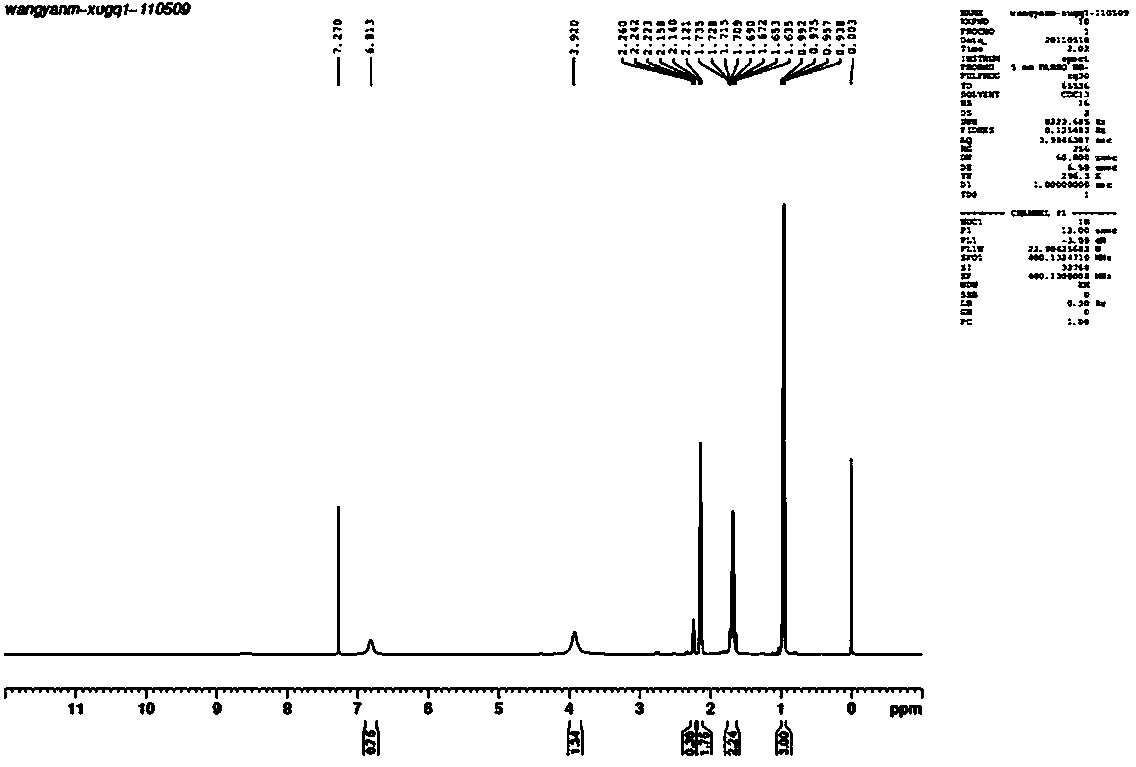
[0040] Example 1 Synthesis of butanoic acid, hydrazide, CAS: 3538-65-6
[0041] Add 116g of ethyl butyrate and 75g of 80% hydrazine hydrate into a three-necked flask equipped with a magnet, and install a thermometer and a rectifying column. Heat the three-neck flask and start stirring, and reflux for half an hour. When the temperature at the top of the rectifying column reaches 80°C, the by-products ethanol and water will distill out. Control the temperature of the reaction system at 82-101°C, and the temperature at the top of the rectifying column at 75-85°C. After 6 hours of reaction, the reaction was completed, and the heating was stopped and stirred. After the reaction was completed, vacuum distillation was performed to distill off excess hydrazine hydrate, water, and a small amount of ethyl butyrate to obtain 93.8 g of hydrazine butyrate product, with a yield of 92%. 1 H NMR (400MHz, CDCl 3 , δ, ppm): 0.94-0.99 (t, 3H, CH 3 ), 1.64-1.74 (sextet, 2H, CH 2 ), 2.12-2.22 (...
Embodiment 2
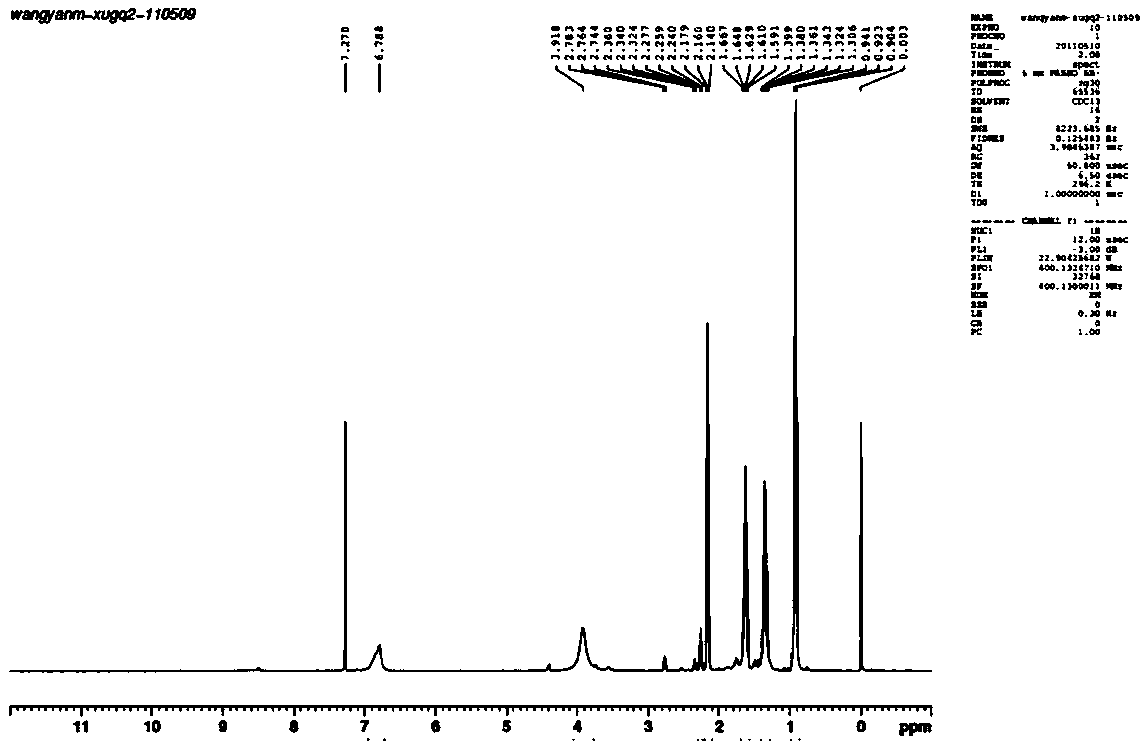
[0042] Example 2 Synthesis of hydrazine pentanoate (Pentanohydrazide, CAS: 38291-82-6)
[0043] Add 130g of ethyl valerate and 75g of 80% hydrazine hydrate into a three-necked flask equipped with a magnet, and install a thermometer and a rectifying column. Heat the three-neck flask and start stirring, and reflux for half an hour. When the temperature at the top of the rectifying column reaches 80°C, the by-products ethanol and water will distill out. Control the temperature of the reaction system at 85-105°C, and the temperature at the top of the rectification column at 75-85°C. After 6 hours of reaction, the reaction was completed, and the heating was stopped and stirred. After the reaction was completed, vacuum distillation was performed to distill off excess hydrazine hydrate, water, and a small amount of ethyl valerate to obtain 106.7 g of hydrazine valerate, with a yield of 92%. 1 H NMR (400MHz, CDCl 3 , δ, ppm): 0.90-0.94 (t, 3H, CH 3 ), 1.31-1.40 (sextet, 2H, CH 2 ...
Embodiment 3
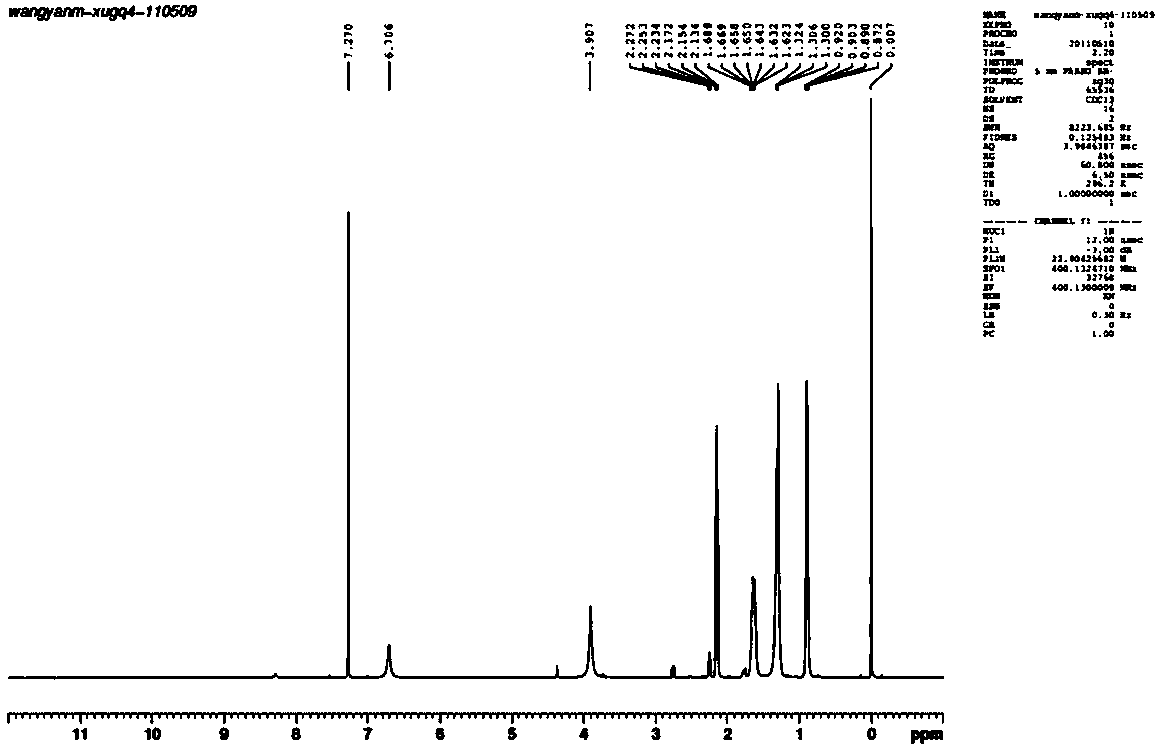
[0044] Example 3 Synthesis of Heptanohydrazide (CAS: 22371-32-0)
[0045] Add 158 g of ethyl heptanoate and 75 g of 80% hydrazine hydrate into a three-necked flask equipped with a magnet, and install a thermometer and a rectifying column. Heat the three-neck flask and start stirring, and reflux for half an hour. When the temperature at the top of the rectifying column reaches 80°C, the by-products ethanol and water will distill out. Control the temperature of the reaction system at 88-112°C, and the temperature at the top of the rectifying column at 75-85°C. After 6 hours of reaction, the reaction was completed, and the heating was stopped and stirred. After the reaction was completed, vacuum distillation was performed to distill off excess hydrazine hydrate, water, and a small amount of ethyl heptanoate to obtain 133.92 g of heptanyl hydrazide product, with a yield of 93%. 1 H NMR (400MHz, CDCl 3 , δ, ppm): 0.87-0.92(t, 3H, CH 3 ), 1.30-1.32(t, 6H, 3CH 2 ), 1.62-1.69 (m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com