Red and blue anthraquinone-contained polyphenylene sulfone copolymers and preparation methods and applications thereof
A technology of anthraquinone polyphenylene sulfone and polyphenylene sulfone, which is applied in the field of polyaryl ether sulfone copolymer preparation, can solve the problems of material mechanical performance degradation, dye decomposition, and high energy consumption, and achieve good solubility and Thermal stability, uniform and stable color, and good transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
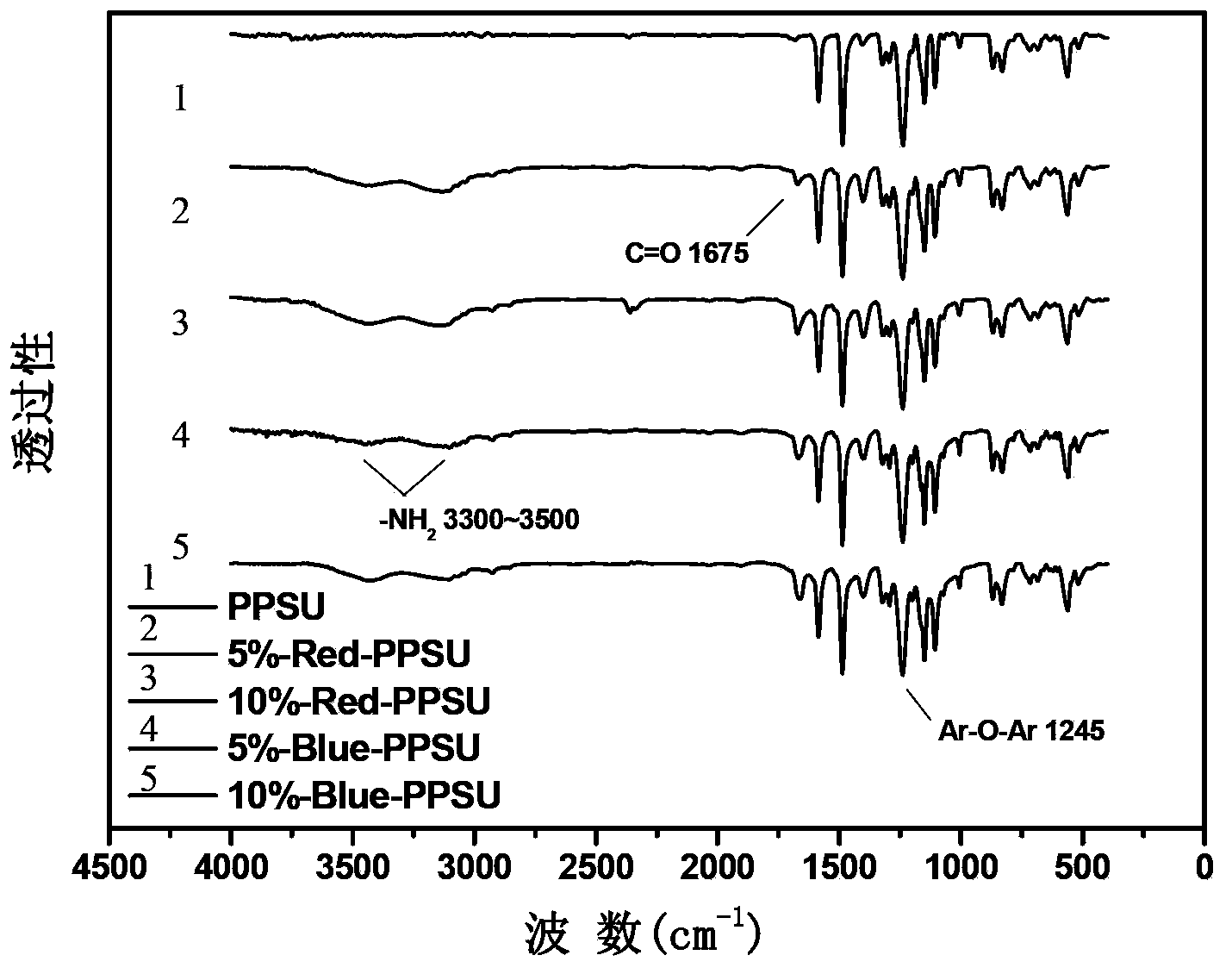
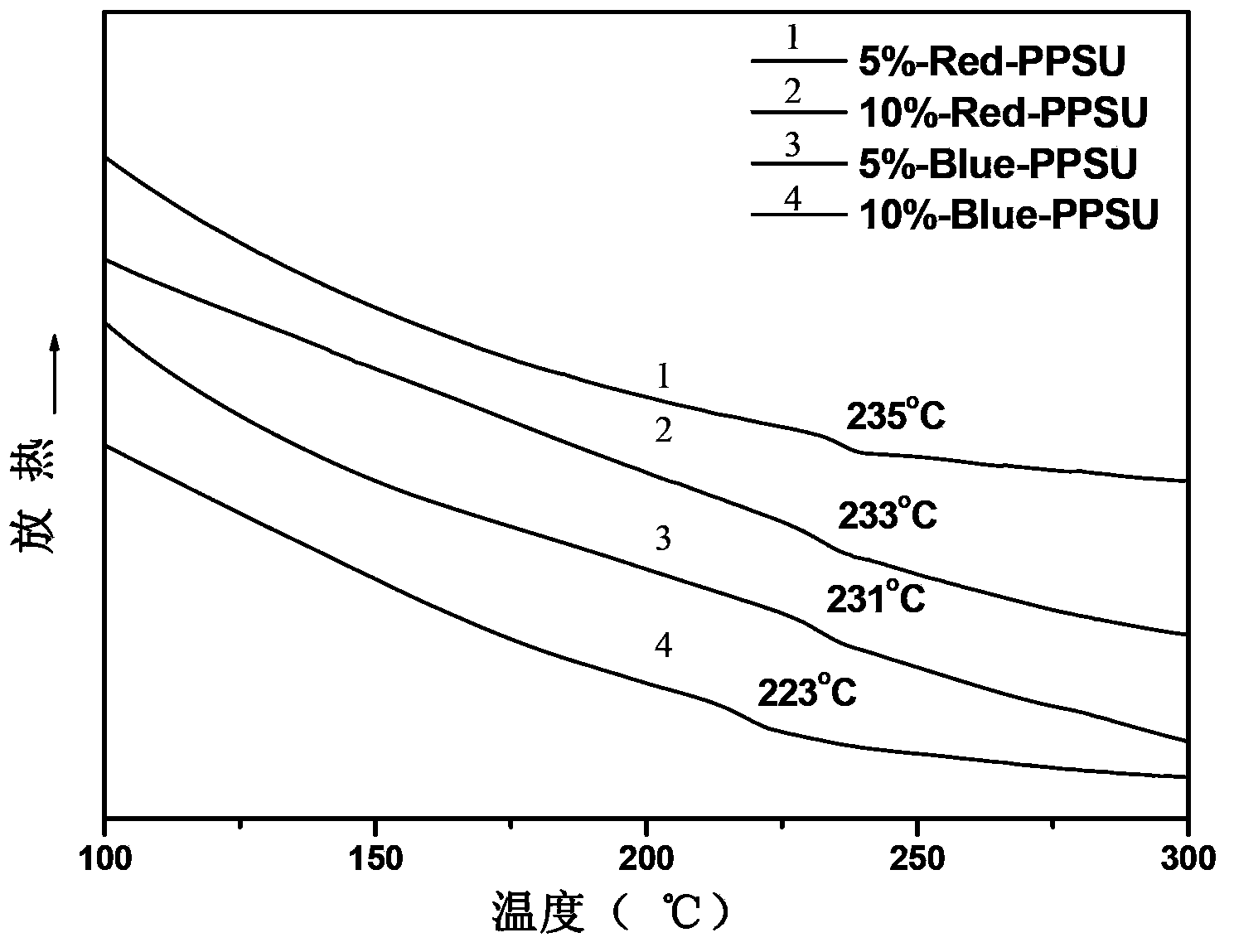
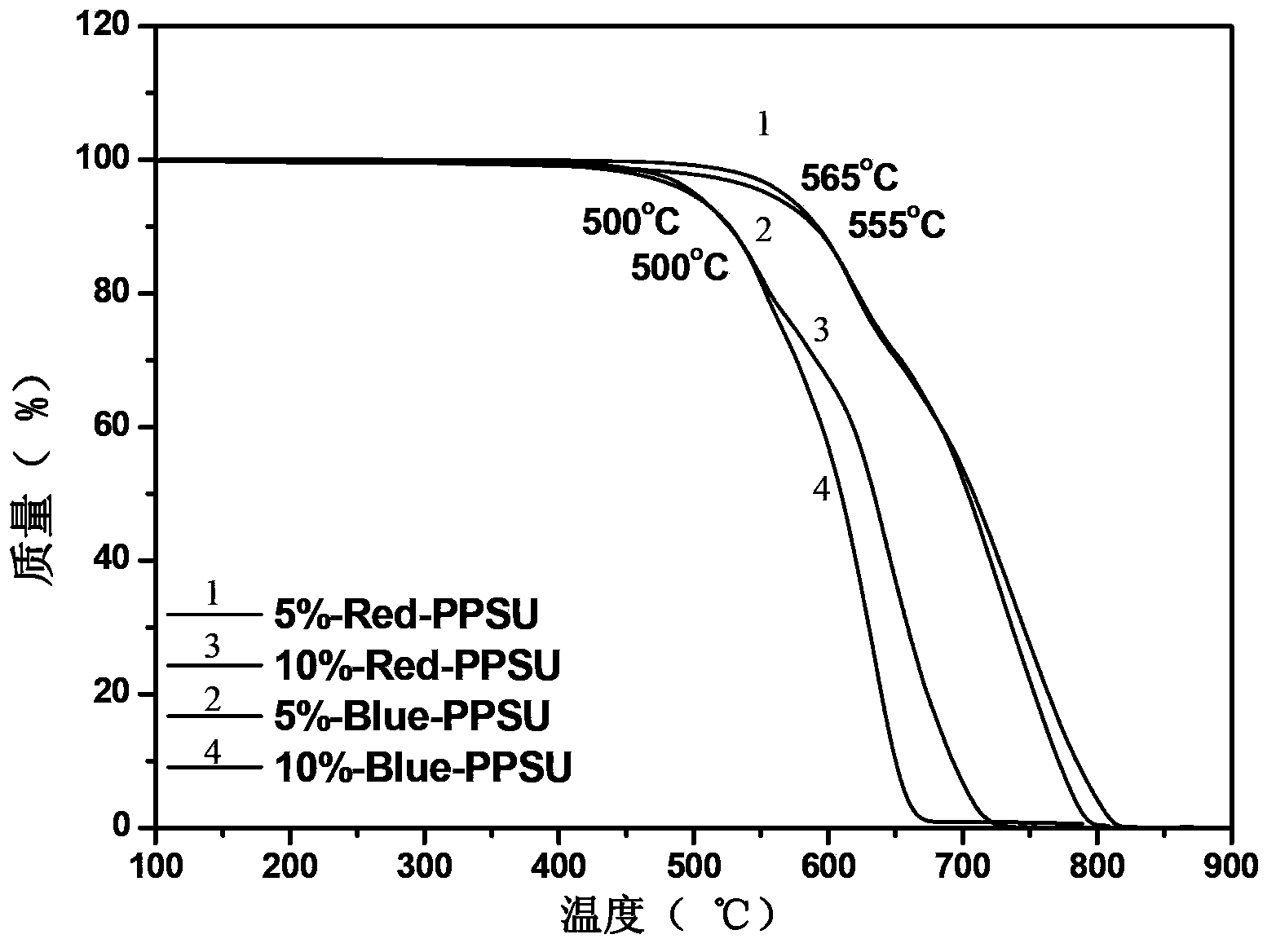
[0035] Add 0.001mol (0.2232g) 1-aminoanthraquinone, 0.020mol (5.0850g) 4,4′-difluorodiphenyl sulfone and 0.0011mol (0.1518g) anhydrous potassium carbonate to a device equipped with mechanical stirring, thermometer, with water In the three-necked flask of the condenser and the condenser, nitrogen protection, 28 mL of sulfolane (TMS) was used as the reaction solvent, the solid content was 20%, and 10 mL of toluene was used as the aqueous agent. Bring water at 140°C for 3 hours, distill out toluene, then gradually heat up from 140°C to 200°C at a rate of 10°C / hour, and stay at 200°C for 2 hours. Heating was stopped, cooled to room temperature, 0.019 mol (3.5380 g) of 4,4'-biphenol and 0.0209 mol (2.8842 g) of anhydrous potassium carbonate were added, and 10 mL of toluene was added again. Bring water at 140°C for 2.5 hours, distill out toluene, then gradually increase the temperature from 140°C to 200°C at a rate of 10°C / hour, and stay at 200°C for 2 hours. Stop heating, when the...
Embodiment 2
[0037] Add 0.003mol (0.6697g) 1-aminoanthraquinone, 0.030mol (7.6275g) 4,4'-difluorodiphenyl sulfone and 0.0033mol (0.4554g) anhydrous potassium carbonate to a device equipped with mechanical stirring, thermometer, with water In the three-necked flask of the condenser and the condenser, nitrogen protection, 43 mL of sulfolane (TMS) was used as the reaction solvent, the solid content was 20%, and 15 mL of toluene was used as the aqueous agent. Bring water at 140°C for 3 hours, distill out toluene, then gradually increase the temperature from 140°C to 210°C at a rate of 10°C / hour, and stay at 210°C for 2 hours. Heating was stopped, cooled to room temperature, 0.019 mol (3.5380 g) of 4,4'-biphenol and 0.0297 mol (2.8842 g) of anhydrous potassium carbonate were added, and 15 mL of toluene was added again. Bring water at 140°C for 3 hours, distill out toluene, then gradually heat up from 140°C to 200°C at a rate of 10°C / hour, and stay at 200°C for 2 hours. Stop heating, when the t...
Embodiment 3
[0039] Combine 0.001mol (0.2702g) 1,5-diamino-4,8-dihydroxyanthraquinone, 0.020mol (5.0850g) 4,4′-difluorodiphenylsulfone and 0.0011mol (0.1518g) anhydrous potassium carbonate Put it into a three-necked flask equipped with a mechanical stirring, a thermometer, a water device and a condenser, nitrogen protection, and use 54 mL of N,N-dimethylacetamide (DMAc) as the reaction solvent, with a solid content of 15%, and 18 mL of toluene as With water. Bring water at 135°C for 3 hours, distill out toluene, then gradually increase the temperature from 135°C to 160°C at a rate of 10°C / hour, and stay at 160°C for 2 hours. Heating was stopped, cooled to room temperature, 0.019 mol (3.5380 g) of 4,4'-biphenol and 0.0209 mol (2.8842 g) of anhydrous potassium carbonate were added, and 18 mL of toluene was added again. Bring water at 135°C for 3 hours, distill out toluene, then gradually increase the temperature from 135°C to 160°C at a rate of 10°C / hour, and stay at 160°C for 2 hours. Sto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com