Exenatide slow release sustained release microsphere, and preparation method and preparation thereof
A technology of exenatide and slow-release microspheres, which is applied in the field of medicine, can solve the problems of exenatide exenatide inactivation, toxicity and immunogenicity, and increase the risk of side effects, so as to achieve optimal drug release Curve, Reduced Aggregation, Enhanced Bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
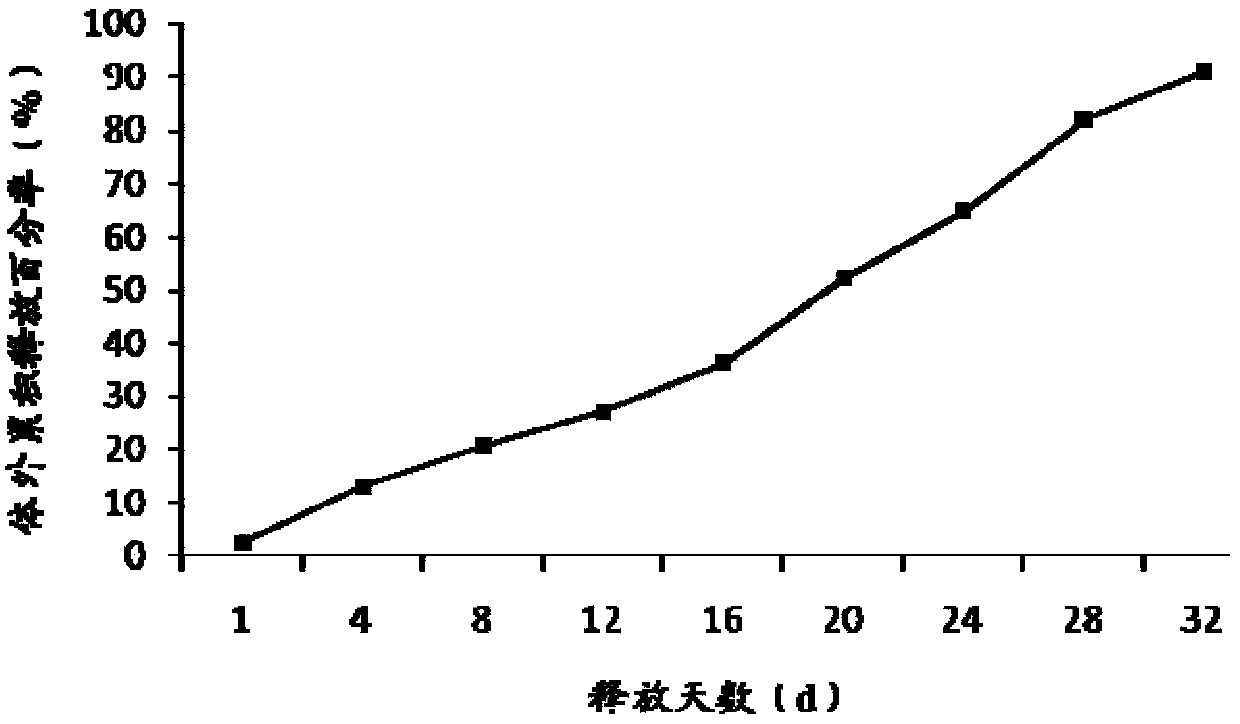
[0048] Embodiment 1 Preparation of Exenatide Sustained-release Microspheres
[0049] Weigh 400mg of exenatide and 60mg of lysine and dissolve them in 4mL of water as the inner water phase; weigh 16g of poly(lactic-co-glycolic acid) copolymer (the ratio of lactide to glycolide is 50:50, 0.2dL / g ) was dissolved in 80 mL of dichloromethane, the inner water phase and the oil phase were vortex mixed for 30 s, and the colostrum was formed by ultrasonic emulsification with an ultrasonic cell disruptor (Branson S-250D) for 1 min under ice bath conditions. Add colostrum into 5000mL of 0.5% polyvinyl alcohol and 1.5% lysine aqueous solution under mechanical stirring to obtain double emulsion, keep stirring this double emulsion for more than 3 hours to volatilize and remove the organic solvent, discard the supernatant after centrifugation, The crude microspheres were obtained, and the exenatide sustained-release microspheres were obtained after washing and drying. After testing, the par...
Embodiment 2
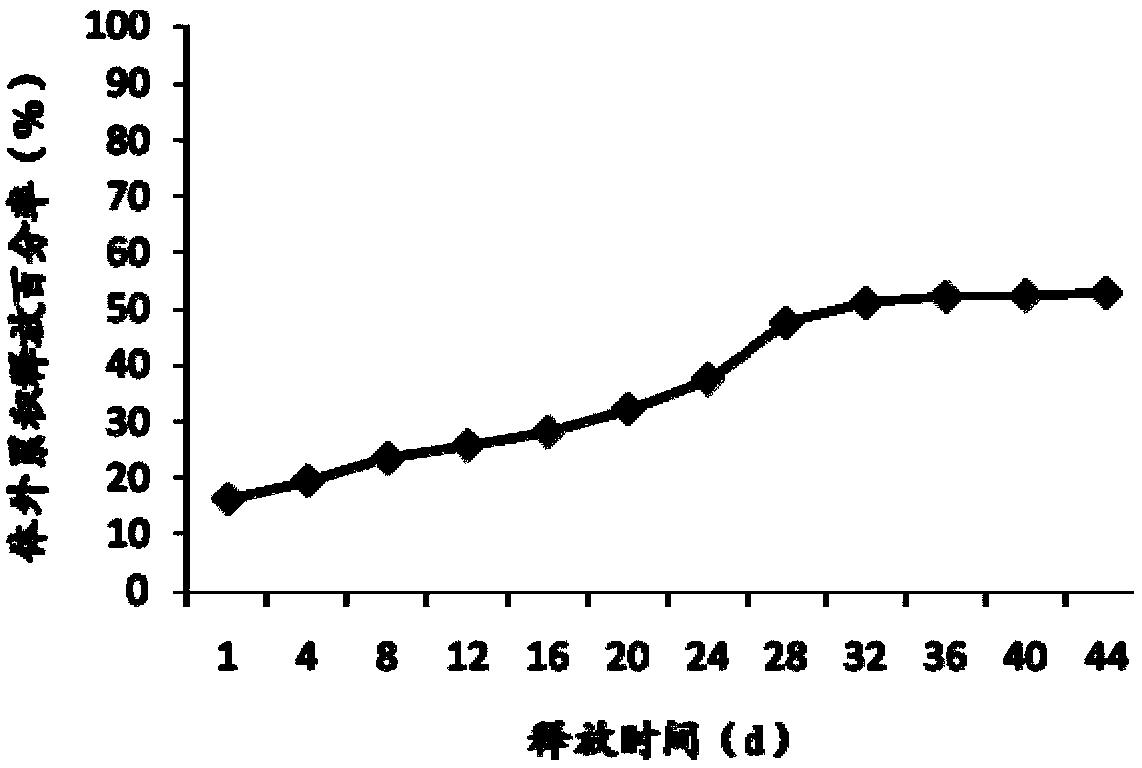
[0050] Embodiment 2 Preparation of Exenatide Sustained-release Microspheres
[0051] Weigh 100 mg of exenatide and 100 mg of glycine, dissolve them in 0.2 mL of water as the inner water phase; weigh 9.9 g of poly(lactic-co-glycolic acid) copolymer (the ratio of lactide to glycolide is 25:75, 0.1dL / g) Dissolve in 19.8 mL of ethyl acetate, vortex mix the inner water phase and oil phase for 30 s, and ultrasonically emulsify for 1 min with an ultrasonic cell disruptor (Branson S-250D) in an ice bath to form colostrum. Add the colostrum into the aqueous solution of 20L of 0.5% polyvinyl alcohol and 10kg of arginine under mechanical stirring to obtain the double emulsion, keep stirring the double emulsion for more than 3 hours to volatilize and remove the organic solvent, discard the supernatant after centrifugation, that is Crude microspheres were obtained, and exenatide sustained-release microspheres were obtained after washing and drying. After testing, the particle size of the...
Embodiment 3
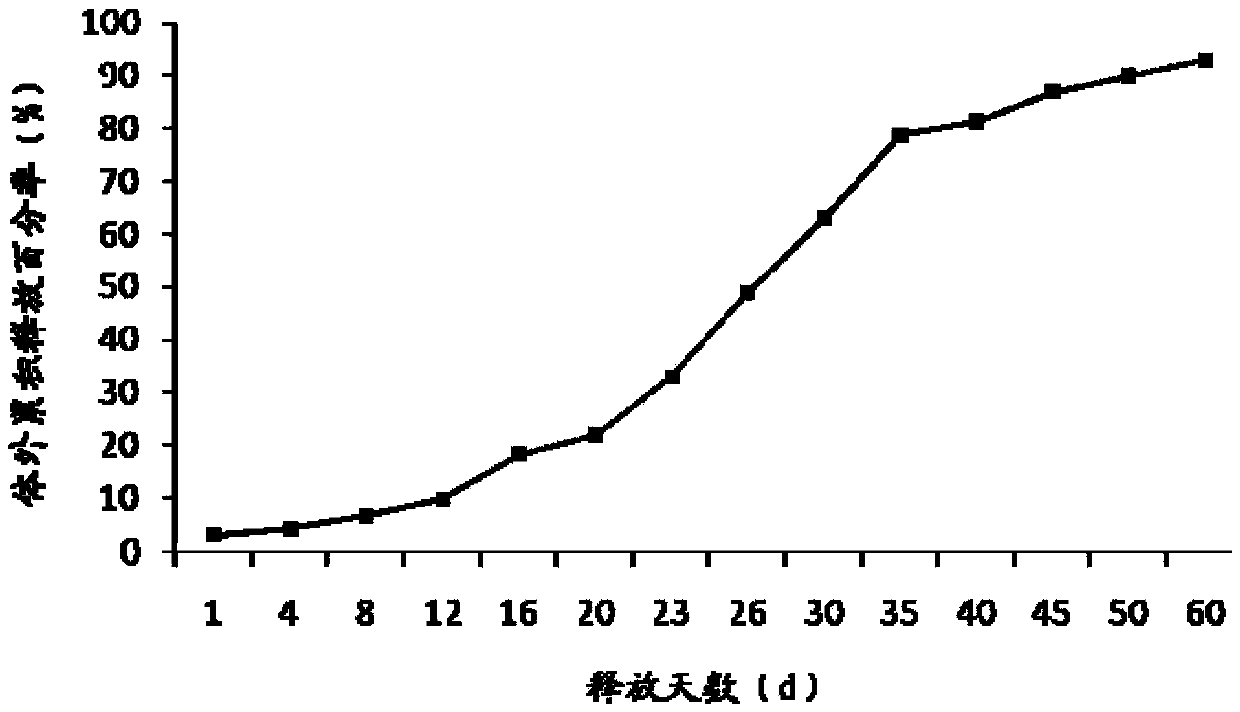
[0052] Embodiment 3 Preparation of Exenatide Sustained-release Microspheres
[0053] Weigh 500 mg of exenatide and 500 mg of histidine, dissolve them in 10 mL of water as the inner water phase; weigh 80 g of poly(lactic-co-glycolic acid) copolymer (the ratio of lactide to glycolide is 75:25, 0.5 dL / g) Dissolve in a mixture of 800mL ethyl acetate and dichloromethane (the volume ratio of ethyl acetate to dichloromethane is 1:1), vortex mix the inner water phase and oil phase for 30s, and use in ice bath Ultrasonic cell breaker (Branson S-250D) phacoemulsification for 1min to form colostrum. Add the colostrum into the aqueous solution of 8.1L 0.1% polyvinyl alcohol and 4.05g histidine under mechanical stirring to obtain the double emulsion, keep stirring the double emulsion for more than 3 hours to volatilize and remove the organic solvent, discard the supernatant after centrifugation, The crude microspheres were obtained, and the exenatide sustained-release microspheres were o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com