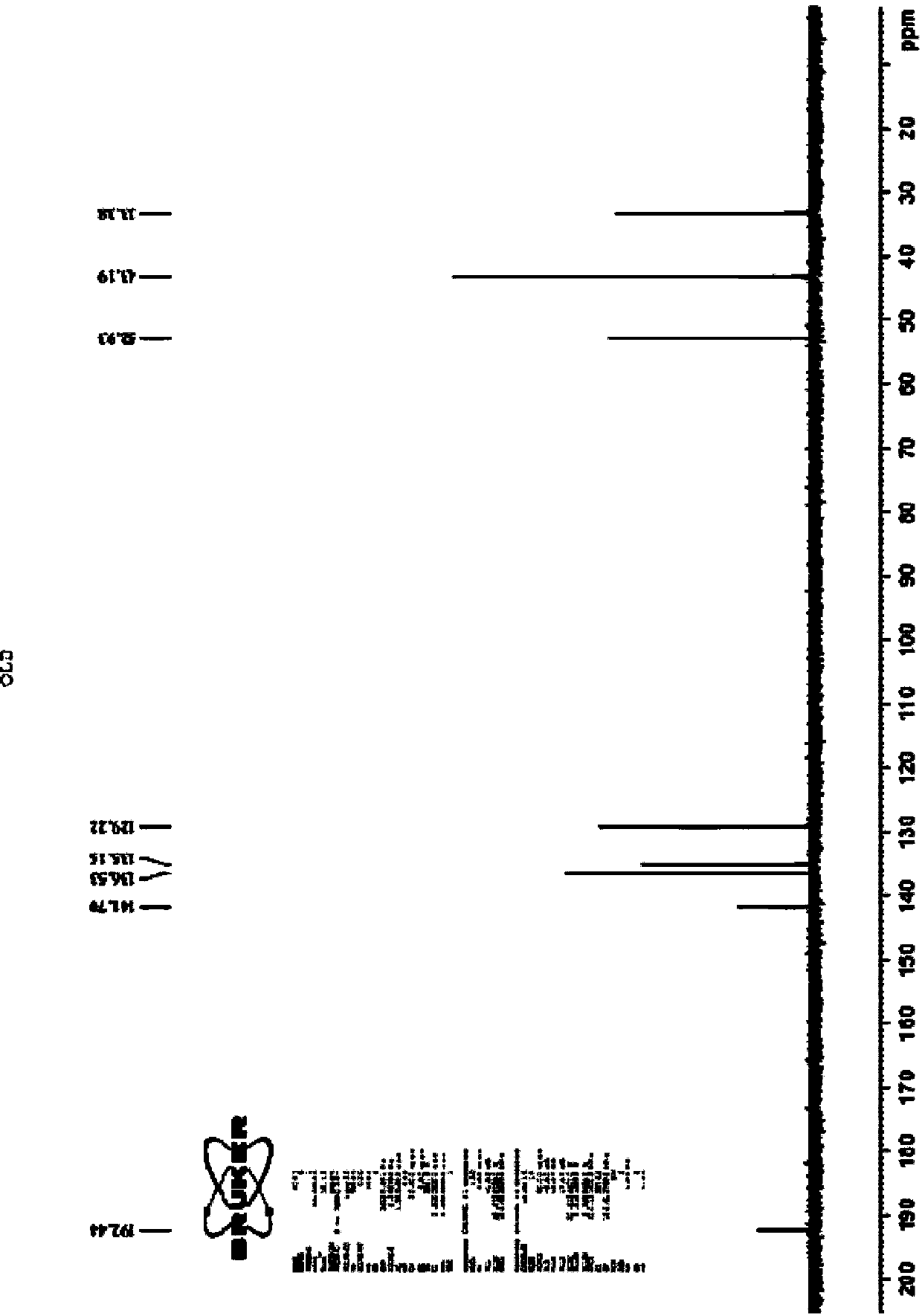
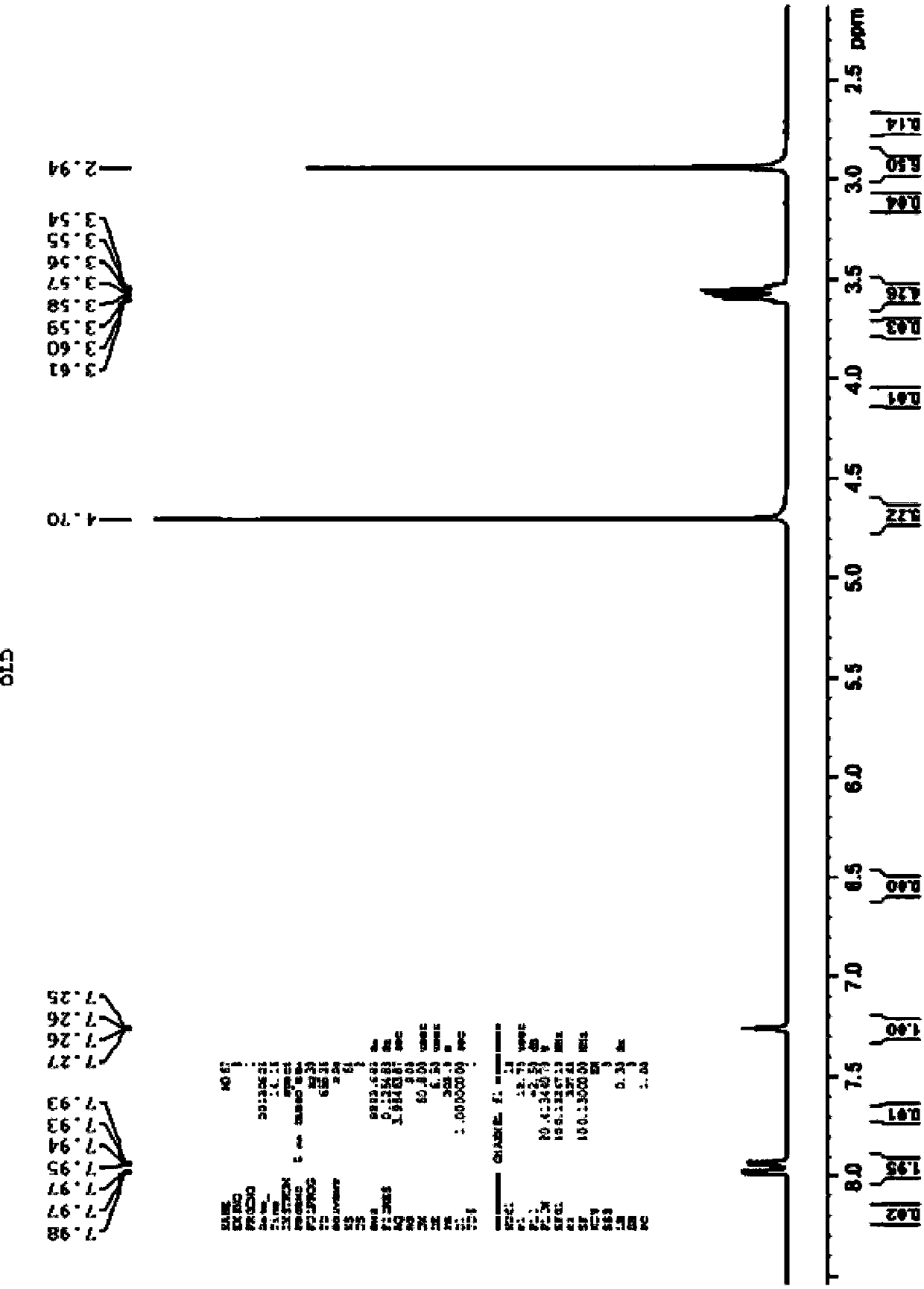
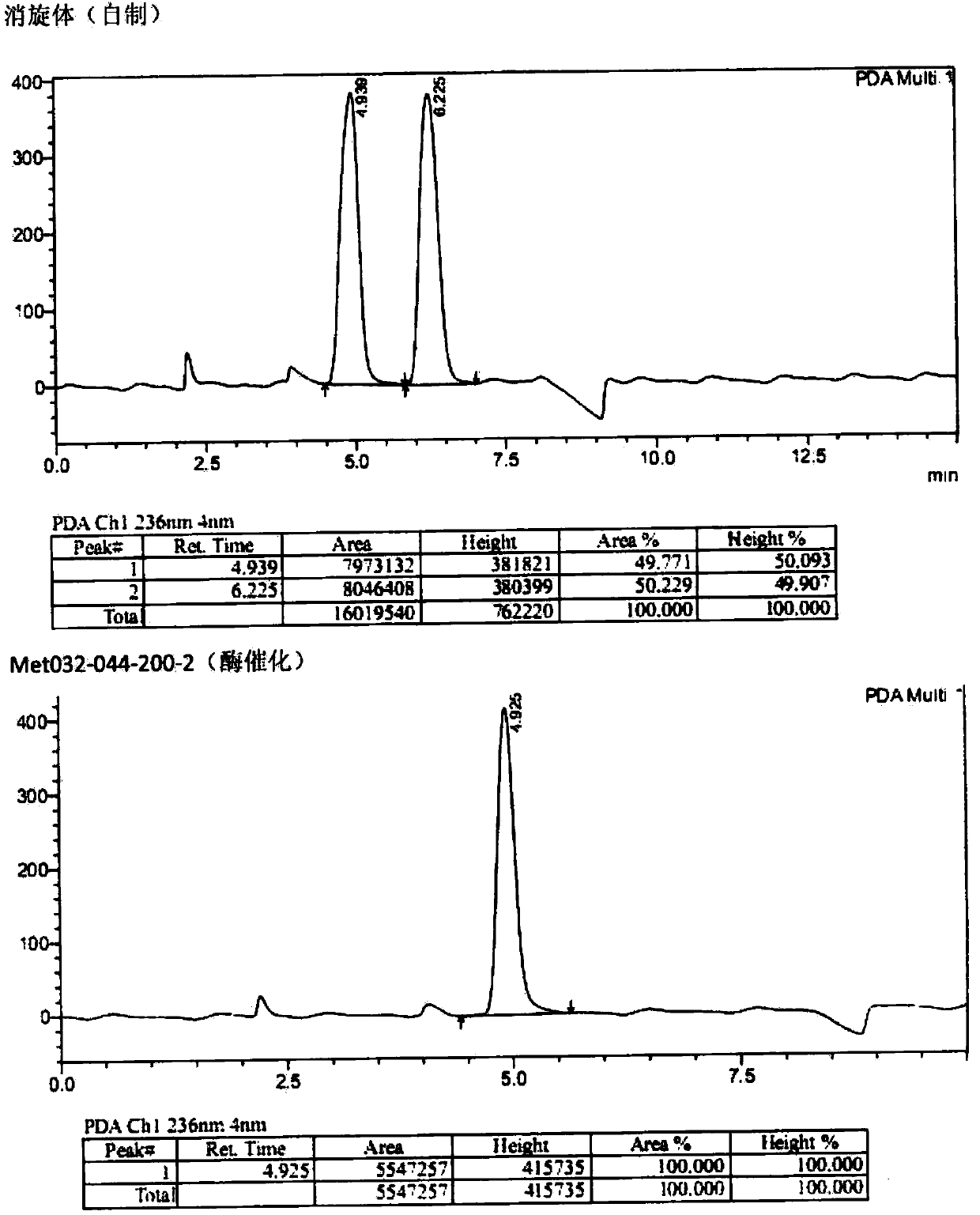
Biological preparation method of (S)-3-(dimethylamino)-1-(thiophene-2-radical)-1-propyl alcohol
A dimethylamine-based, biological preparation technology, applied in the direction of fermentation, can solve the problems of complicated operation, difficult to repeat, and flammability, and achieve the effect of strong process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention provides a method for the biological preparation of (S)-3-(dimethylamino)-1-(thiophen-2-yl)-1-propanol by using a combination of ketoreductase and glucose reductase, and the reaction equation as follows:
[0022]
Embodiment 1
[0025] Add 1.0 g of substrate and 1.5 g of glucose to a 50 mL three-necked reaction flask, and then add 10 mL of triethanolamine buffer solution with a pH of 7.0 prepared in advance to the reaction flask. Stir in a magnetic stirring water bath at 30°C, and adjust the pH to 7.0 with 10wt% sodium carbonate solution. After the temperature stabilized, 20 mg of ketoreductase, 40 mg of glucose dehydrogenase and 10 mg of NADP were added to the reaction bottle to start the reaction, and the reaction temperature was maintained at 30°C. The pH of the reaction solution was adjusted by adding sodium carbonate solution dropwise with a pH titrator. Control the pH at 7.0. Regular sampling for HPLC detection central control. React for 22-24 hours, conversion rate > 98%, adjust the reaction solution to alkaline with concentrated sodium hydroxide solution, filter with diatomaceous earth, add toluene for extraction, combine the organic phase, dry the organic phase with anhydrous sodium sulfate...
Embodiment 2
[0027] Add 10 g of substrate and 15 g of glucose into a 500 mL three-necked reaction flask, and then add 100 mL of a pre-prepared triethanolamine buffer solution with a pH of 7.0 into the reaction flask. Stir in a magnetic stirring water bath at 30°C, and adjust the pH to 7.0 with 10wt% sodium carbonate solution. When the temperature is stable, add 200 mg ketoreductase, 400 mg glucose dehydrogenase and 100 mg NADP into the reaction flask, and the reaction starts. The reaction temperature was maintained at 30°C. The pH of the reaction solution was adjusted by adding sodium carbonate solution dropwise with a pH titrator, and the pH was controlled at 7.0. Regular sampling for HPLC detection central control. React for 22-24 hours, conversion rate > 98%, adjust the reaction solution to alkaline with concentrated sodium hydroxide solution, filter with diatomaceous earth, add toluene for extraction, combine the organic phase, dry the organic phase with anhydrous sodium sulfate, con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com