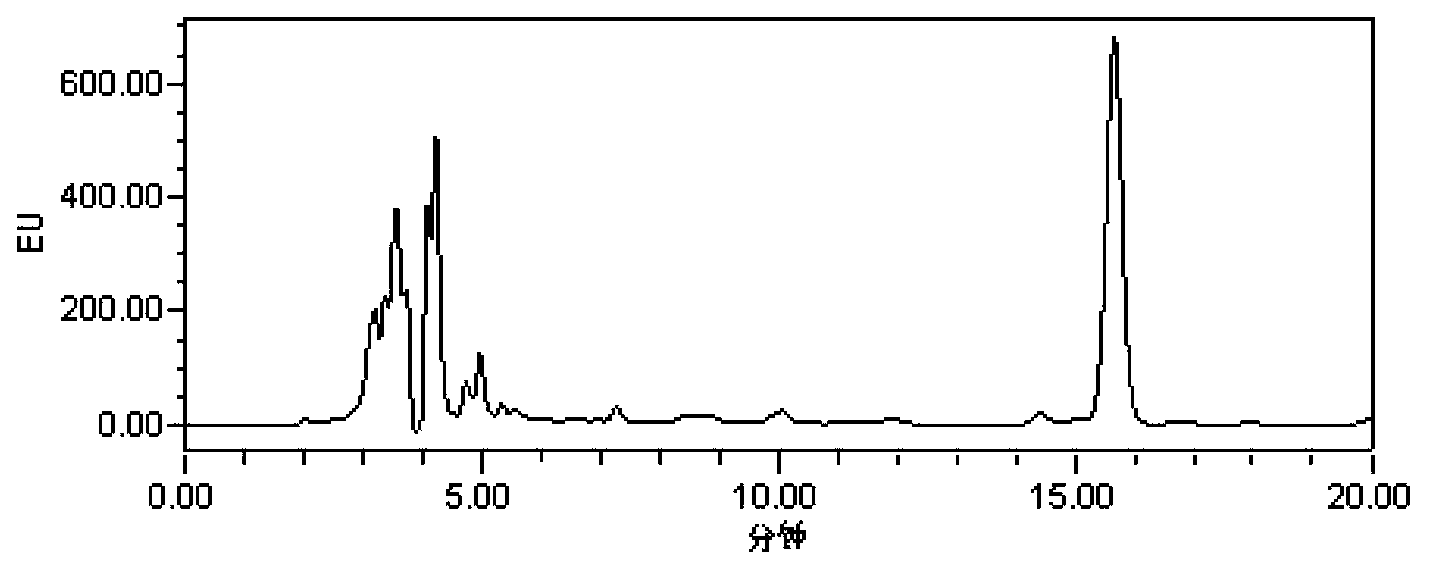
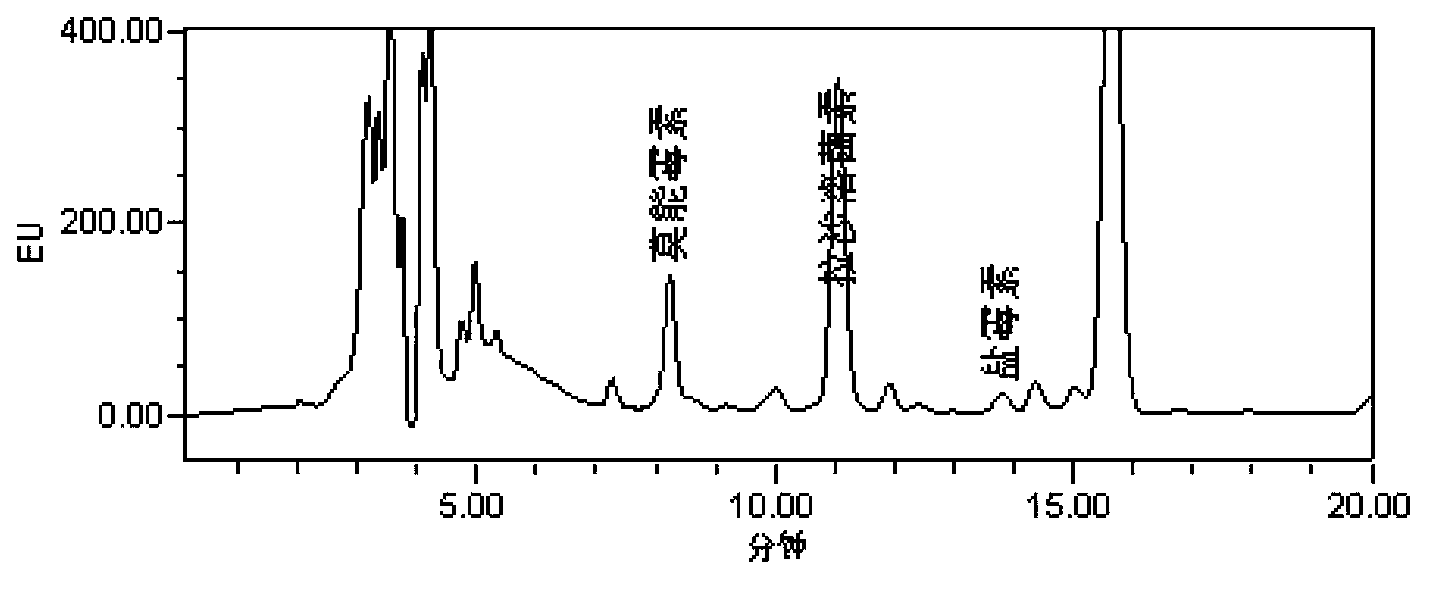
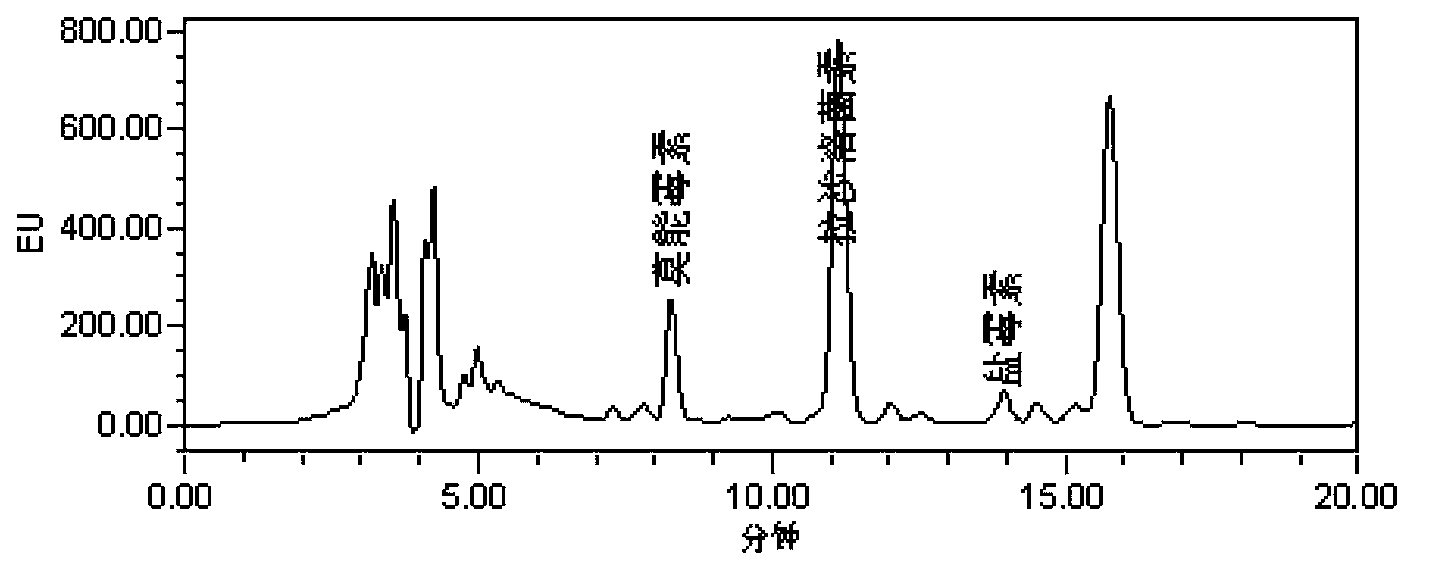
Analysis method of monensin, salinomycin and lasalocid residues
A detection method and drug technology, applied in the direction of analyzing materials, material separation, measuring devices, etc., to achieve the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the derivatization reaction of carboxyl compound and bromoacetylpyrene
[0048] (1) Monensin sodium, salinomycin sodium, Lasalocid (Lasalocid A sodium salt) derivatization reaction with bromoacetylpyrene
[0049] Accurately weigh an appropriate amount of monensin sodium, salinomycin sodium, and 100 mg of the reference substance of lasalocid (lasalocid A sodium salt), dissolve it in methanol into a 10 mL volumetric flask, and prepare a 10 mg / mL standard respectively. stock solution. Accurately draw 1500 μL, 600 μL, and 400 μL of standard stock solutions of monensin sodium, salinomycin sodium, and lasalocid (Lasalocid A sodium salt) into 10 mL volumetric flasks, dilute to the mark with acetonitrile, and mix well That is, (concentration: 1.5mg / mL, 0.6mg / mL, 0.4mg / mL, respectively). Take 1mL from each volumetric flask, put it into a 10mL volumetric flask, and dilute to the mark with acetonitrile to obtain mixed standard working solutions with respective conc...
Embodiment 2
[0062] Embodiment 2: sample determination
[0063] (1) Processing of samples
[0064] The muscle, liver and kidney tissues of chicken and pig were selected as experimental samples, and the addition and recovery experiment of monensin, salinomycin and lasalocid was carried out. Add an appropriate amount of standard solution to the blank sample, so that the concentration in the sample is LOQ, 1MRL, and 2MRL respectively (the specific concentration of each drug is shown in the table below), and the sample is tested after pretreatment. Repeat 5 samples for each concentration on the same day, calculate the recovery rate, and take the average value to calculate the intra-day coefficient of variation. Each tissue was repeated for 5 days, and the average recovery rate was calculated.
[0065] Extraction: Accurately weigh (5±0.05) g of fresh or thawed sample and place it in a centrifuge tube, add 15 mL of isooctane, vortex for 2 min, centrifuge at 8000 r / min for 10 min, take out the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com