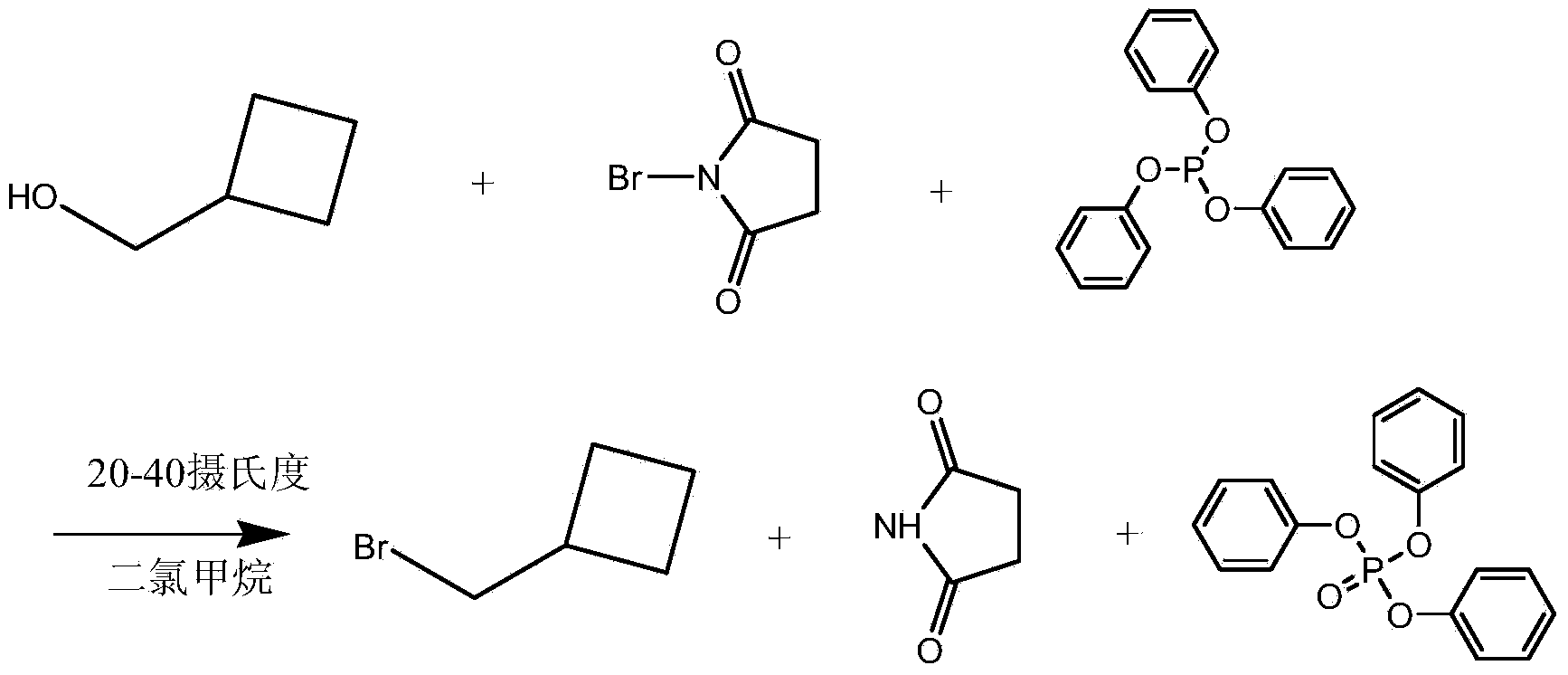
Preparation method of bromomethyl cyclobutane
A technology of bromomethylcyclobutane and cyclobutane, which is applied in the field of preparation of analgesic drug intermediates, can solve the problems of high environmental protection pressure and high cost, and achieve the effects of easy operation, cheap raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 86g of cyclobutanol (1mol), 372g (1.2mol) of triphenyl phosphite and 860mL of dichloromethane into a 2-liter reaction flask equipped with a stirrer and a thermometer, and add 214g of bromosuccinyl in batches under stirring Imine (1.2mol), control the reaction temperature at 38±2°C, after the addition, continue to stir at constant temperature for 4 hours. After the reaction is completed, the organic phase is washed with water four times, each time with 50 mL of water, and then concentrated by atmospheric distillation. After the dichloromethane has been removed, it is then distilled under reduced pressure. Rectification, cooling the condenser with ice water, collecting the fraction at 134-136°C to obtain 108g of bromomethylcyclobutane, that is, the yield is 72%, and analyzed by liquid chromatography, the purity of the prepared bromomethylcyclobutane is 98.3%.
Embodiment 2
[0029] Add 430g cyclobutanol (5mol), 1706g (5.5mol) of triphenyl phosphite and 4L dichloromethane into a 10-liter reaction flask equipped with a stirrer and a thermometer, and add 979g bromosuccinyl in batches under stirring Imine (5.5mol), control the reaction temperature at 35±2°C, after the addition, continue to stir at constant temperature for 6 hours. After completion of the reaction, the organic phase was washed with water four times, each time with 1500 mL of water, then distilled at atmospheric pressure, then distilled under reduced pressure, cooled the condenser with ice water, collected all the cuts, then carried out rectification at normal pressure, cooled the condenser with ice water, The fractions at 134-136°C were collected to obtain 559g of bromomethylcyclobutane, that is, the yield was 75%. Through liquid chromatography analysis, the purity of bromomethylcyclobutane was 98.6%.
Embodiment 3
[0031] Add 86g of cyclobutanemethanol (1mol), 573g (1.5mol) of monophenylenedioctyl phosphite (1.5mol) and 600mL of diethyl ether into a 2-liter reaction flask equipped with a stirrer and a thermometer, and add 515g of dibromohydantoin in batches under stirring (1.8mol), control the reaction temperature at 38±2°C, after the addition, continue to stir at constant temperature for 4 hours. After the reaction is complete, the organic phase is washed four times with 50 mL of water each time, and then concentrated by atmospheric distillation. After the ether has been removed, it is then distilled under reduced pressure, and the condenser is cooled with ice water. All fractions are collected, and then subjected to atmospheric distillation. , cooling the condensing tube with ice water, collecting the distillate at 134-136°C to obtain 109g of bromomethylcyclobutane, that is, the yield was 73%, and analyzed by liquid chromatography, the purity of bromomethylcyclobutane was 98.2 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com