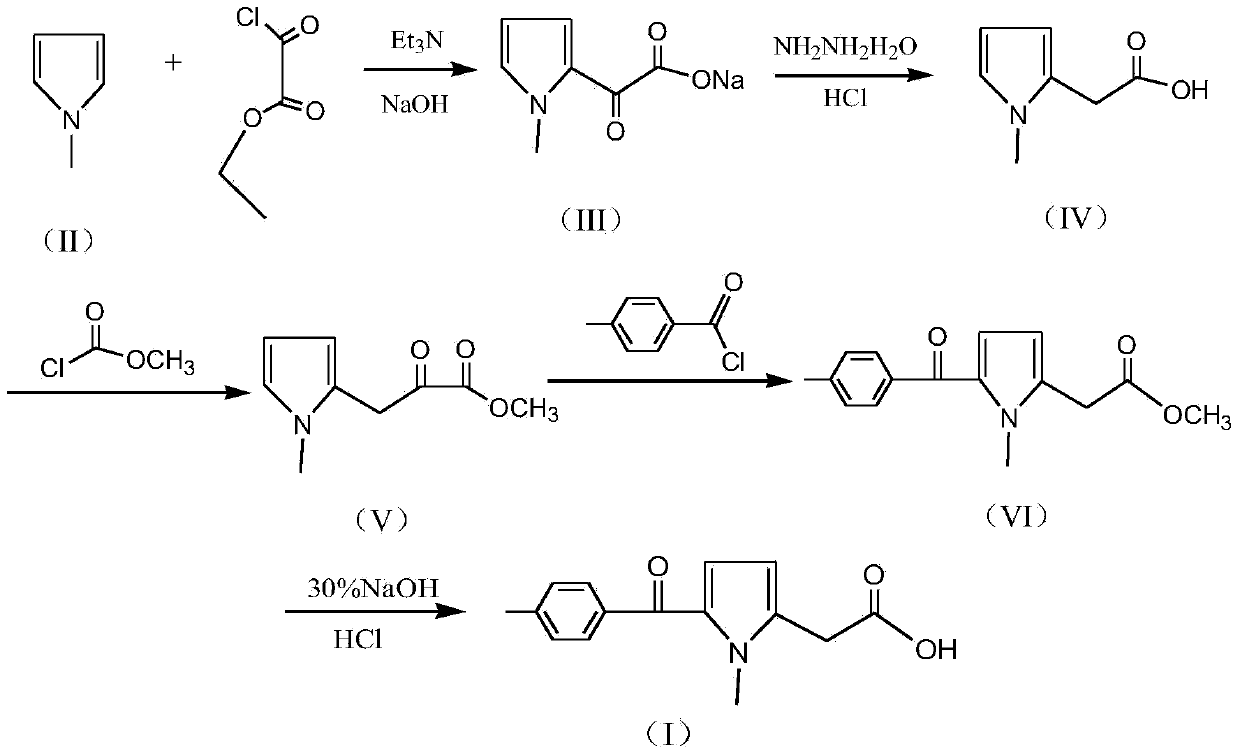
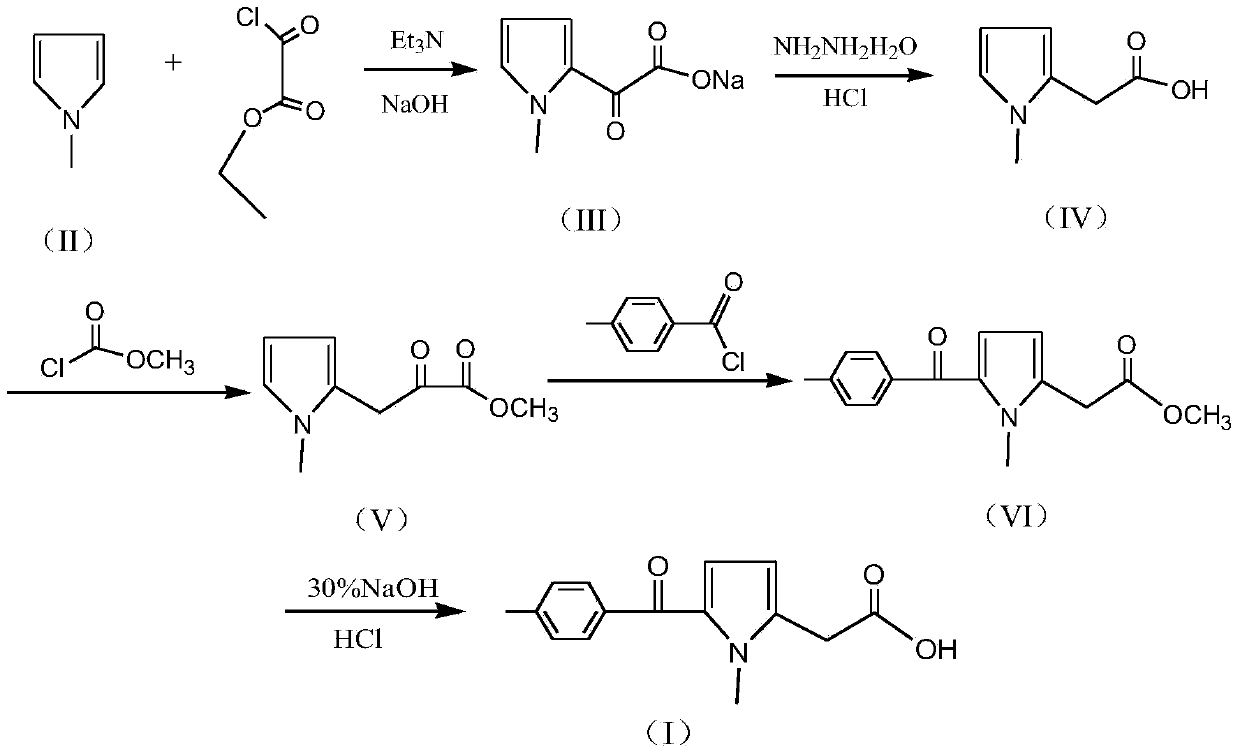
Preparation method of non-steroid anti-inflammatory drug tolmetin
The technology of a non-steroidal anti-inflammatory drug and tolmetine is applied in the preparation of non-steroidal anti-inflammatory drugs and the field of preparation of non-steroidal anti-inflammatory drug tolmetine, and can solve the problems that raw materials are not easily available, the total yield is low, and the It is difficult to obtain and other problems to achieve the effect of avoiding production and operation environment problems, reducing production costs, avoiding pollution problems and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Synthesis of (1-methyl-1H-pyrrol-2-yl)-oxo-acetate sodium (III): In a 2000ml flask, add 600g of toluene, 100g of N-methylpyrrole and 160g of triethylamine, After stirring and mixing for 30 minutes, start to slowly add 190g monoethyl oxalyl chloride dropwise, control the temperature 30±3°C during the dropwise addition, and add the dropwise time for 2 hours. and 375g of water, heated to an internal temperature of 70±5°C, stirred and reacted for 2 hours, then cooled to room temperature and allowed to stand for layering to obtain an aqueous layer solution (toluene layer is distilled to recover toluene).
[0026] 2) Synthesis of N-methylpyrrole-2-acetic acid (IV): in the (1-methyl-1H-pyrrol-2-yl)-oxo-acetate sodium (III) aqueous layer solution obtained in step 1) , add 80% hydrazine hydrate 100g and 60g sodium hydroxide solid, heat to 80±3°C and keep it for 2 hours, then slowly heat up to 100°C within 1 hour, and keep it at 100±2°C for 5 hours; cooling reaction solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com