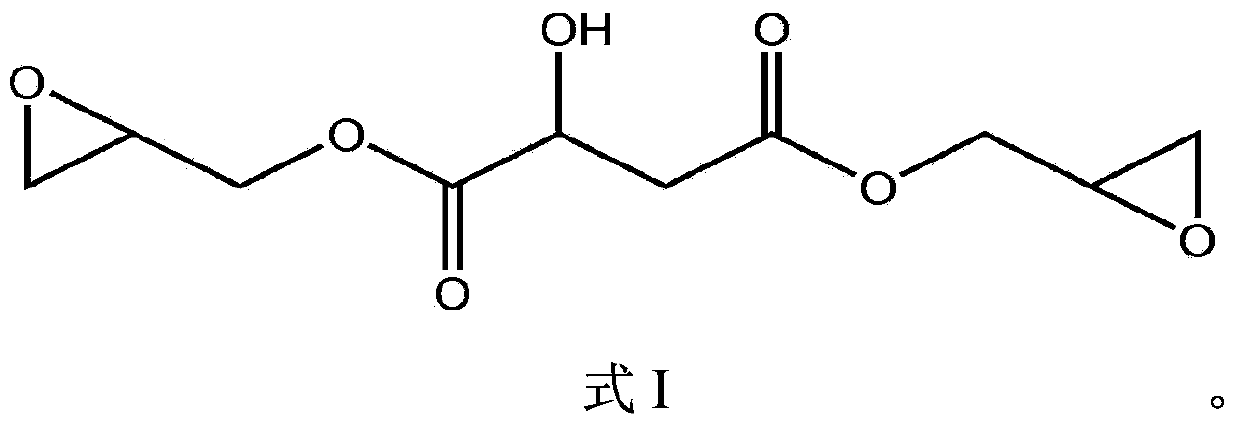
Malic acid radical water-borne epoxy resin and preparation method thereof
A water-based epoxy resin, malic acid-based technology, applied in organic chemistry and other directions, can solve unseen problems and achieve the effect of saving resources, good glass transition temperature, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Mix 100g malic acid, 1200g dioxane, 800g allyl chloride and 60g sodium hydroxide, react at 80°C for 8 hours, filter, wash with water, distill under reduced pressure to remove solvent, water, etc. to obtain diallyl malate base ester.
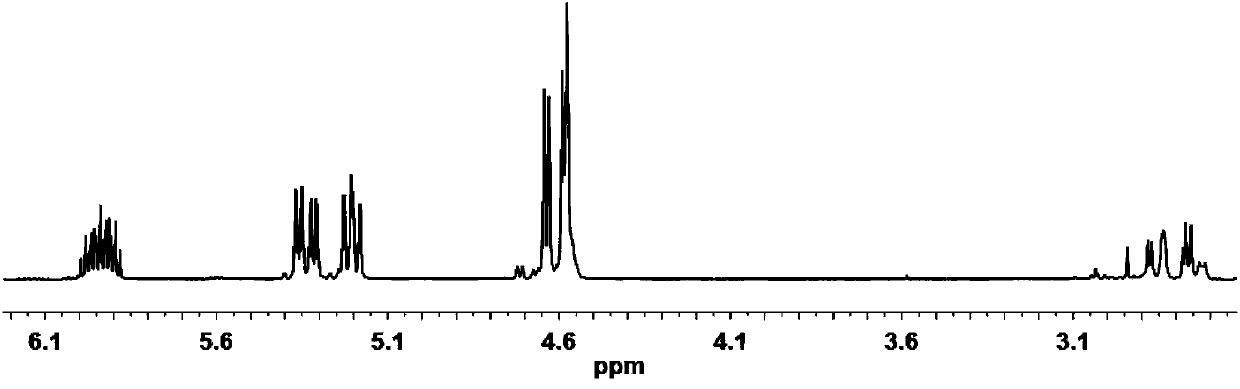
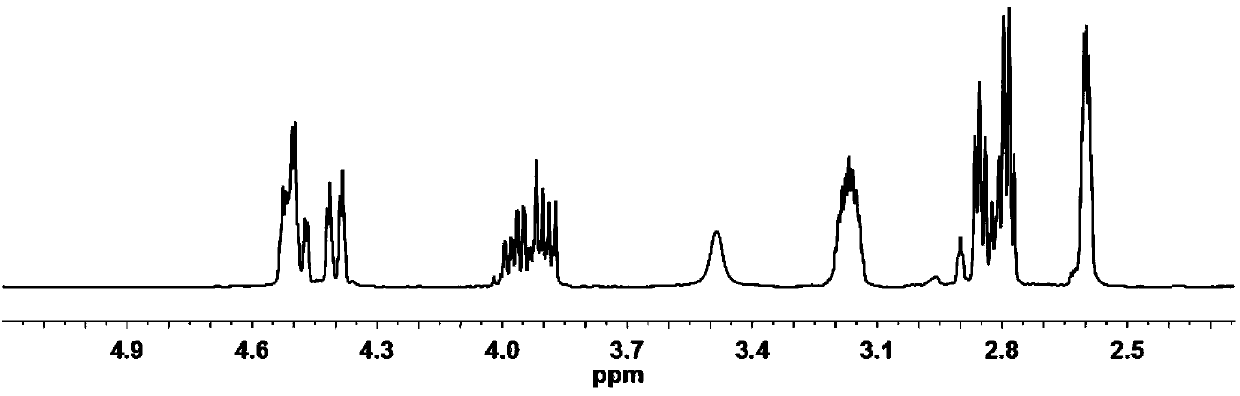
[0034] The diallyl malate of 1 H NMR spectrum as figure 1 as shown, figure 1 The appearance of 5.88-5.97ppm and 5.18-5.37ppm in the middle represents the H on the allyl double bond, about 4.57-4.66ppm represents the H added to the methylene adjacent to the double bond on the allyl group, and 2.84ppm represents - The H on OH, and other peaks are consistent with the H proton shift of diallyl malate, which proves that the obtained diallyl malate has the structure of formula II.
[0035]
[0036] 2) Mix 100g of diallyl malate prepared above, 30g of hydrogen peroxide, 20g of formic acid, 5g of Amberlite-120 (Amberlite series ion exchange resin produced by Rohm and Haas Company) and 50g of dioxane at 60 After reacting at ℃ for 60 hours...
Embodiment 2
[0041] 1) Mix 100g malic acid, 900g tetrahydrofuran, 400g allyl chloride and 400g potassium carbonate, react at 40°C for 72 hours, filter, wash with water, distill under reduced pressure to remove solvent, water, etc. to obtain diallyl malate.
[0042] According to the diallyl malate 1The H NMR spectrum shows that 5.88-5.97ppm and 5.18-5.37ppm in the figure represent the H on the allyl double bond, and about 4.57-4.66ppm represents the methylene adjacent to the double bond on the allyl plus H, 2.84ppm represents the H on the -OH, and other peaks are consistent with the H proton displacement of diallyl malate, which proves that the obtained diallyl malate has the structure of formula II.
[0043]
[0044] 2) Mix 100g of diallyl malate prepared above, 700g of m-chloroperoxybenzoic acid and 2500g of dichloromethane, react at 25°C for 200 hours, then wash 5 times with 5% sodium sulfite aqueous solution, and then Wash with 15% sodium carbonate aqueous solution for 5 times, then...
Embodiment 3
[0049] 1) Mix 100g malic acid, 100g dimethyl sulfoxide, 300g allyl bromide and 300g 1,8-diazabicyclo[5.4.0]undec-7-ene, react at 0℃ for 0.5 hours, Filtrate, wash with water, and distill off the solvent and water under reduced pressure to obtain diallyl malate.
[0050] According to the diallyl malate 1 The H NMR spectrum shows that 5.88-5.97ppm and 5.18-5.37ppm in the figure represent the H on the allyl double bond, and about 4.57-4.66ppm represents the methylene adjacent to the double bond on the allyl plus H, 2.84ppm represents the H on the -OH, and other peaks are consistent with the H proton displacement of diallyl malate, which proves that the obtained diallyl malate has the structure of formula II.
[0051]
[0052] 2) Mix 100g of diallyl malate prepared above, 400g of hydrogen peroxide, 120g of formic acid, 180g of sulfuric acid and 900g of butanone, react at 70°C for 10 hours, and then wash with 20% by mass aqueous solution of sodium dithionite 1 time, then wash w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com