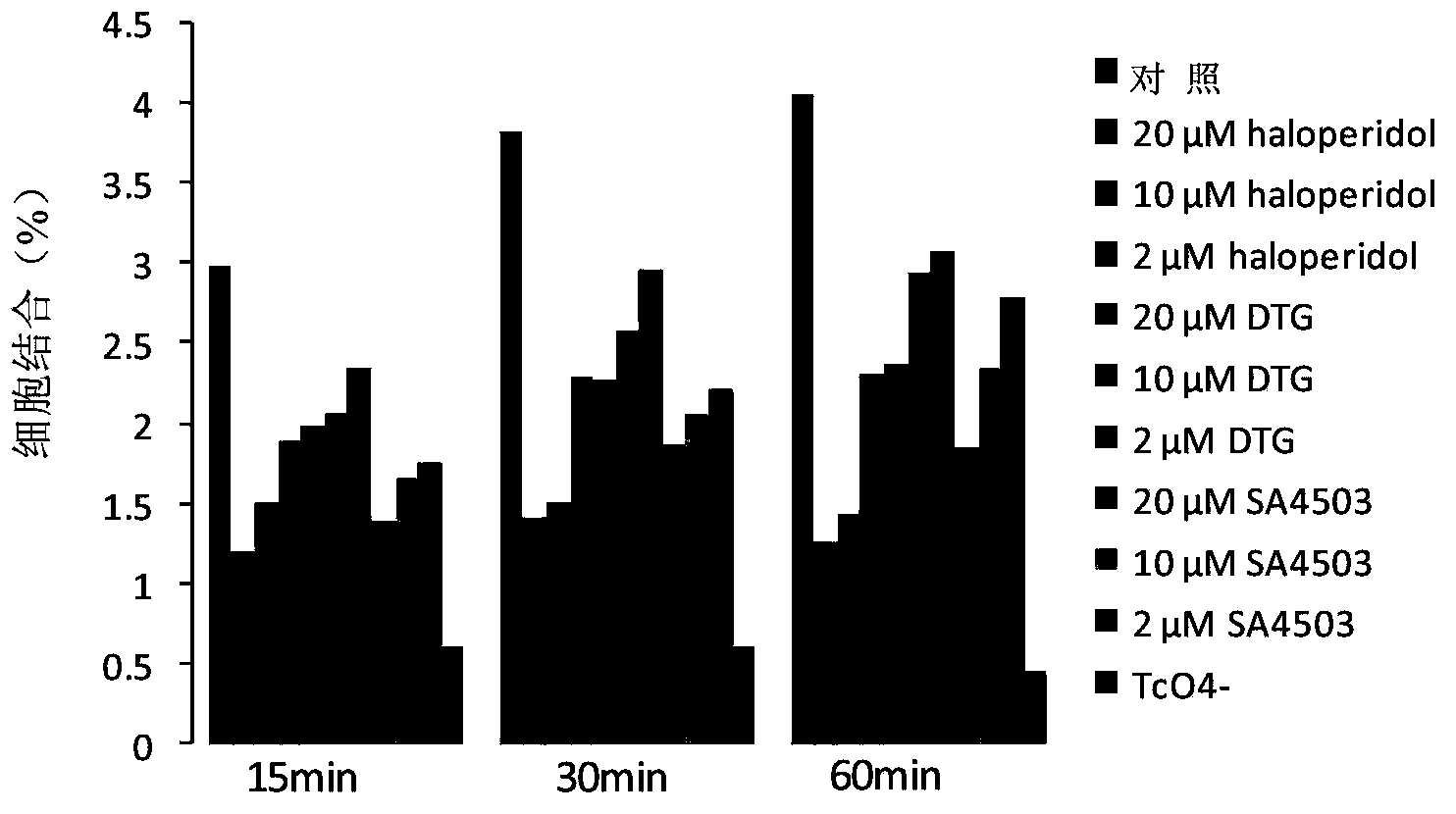
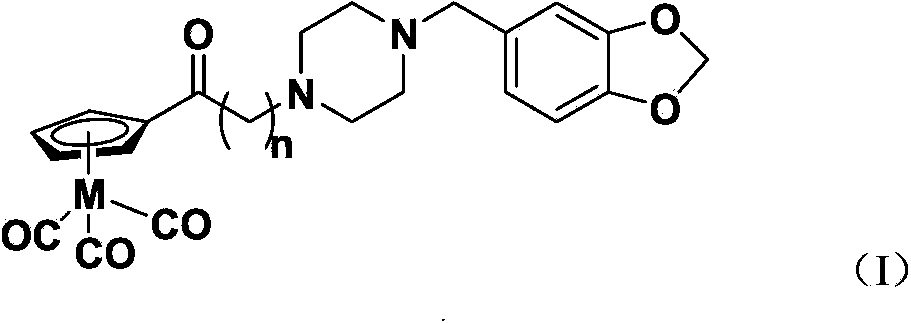
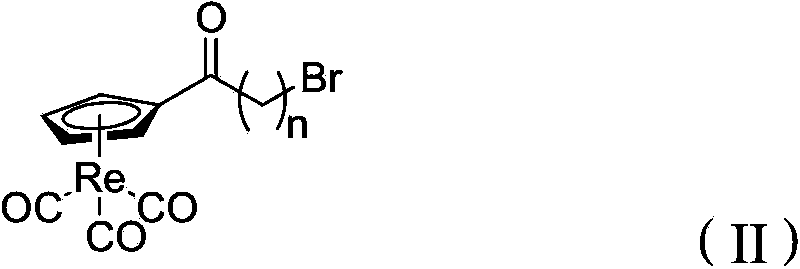Sigma 1 receptor bound tricarbonyl cyclopentadiene ligand compound, and preparation method and application of ligand compound
A technology of tricarbonyl cyclopentadiene and ligand compounds, applied in the fields of radiopharmaceutical chemistry and clinical nuclear medicine, can solve the problems of less research on SPECT imaging agents, and achieve excellent biological properties, high radiochemical purity, high The effect of tagging rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Combining σ 1 Synthesis of Tricarbonylcyclopentadiene Ligand Compound (Re-4-W) of Receptor
[0037]Dissolve 55.0 mg of Re-4-Br (4-bromobutyrylcyclopentadienetricarbonylrhenium) and 28.4 mg of 1-(3,4-methylenedioxybenzyl) piperidine in 2 mL of toluene and 2 mL of triethylamine To the solution, 9.1 mg of potassium iodide was added, stirred in the dark, and refluxed at 115° C. for 4 h. The solvent was spun off, and purified by a 200-300 mesh silica gel column, using ethyl acetate:petroleum ether:triethylamine=1:5:1 (volume ratio) as eluent to obtain 11.2mg of orange-red liquid (yield 15.7% ), the compound Re-4-W. The structure of the product was confirmed by nuclear magnetic resonance analysis and high-resolution mass spectrometry, the results: 1 H NMR (400MHz, CDCl 3 )δ: 6.83(s, 1H), 6.73(s, 2H), 5.97(t, J=2.1Hz, 2H), 5.93(s, 2H), 5.38(t, J=2.1Hz, 2H), 3.39( s, 2H), 2.60 (t, J = 6.9 Hz, 2H), 2.42-2.33 (m, 10H), 1.90-1.83 (m, 10H). 13 C NMR (100MHz, CDCl 3...
Embodiment 2
[0040] Example 2 Combining σ 1 Synthesis of Tricarbonylcyclopentadiene Ligand Compound (Re-6-W) of Receptor
[0041] Dissolve 66.3 mg of Re-6-Br (6-bromohexanoylcyclopentadienetricarbonylrhenium) and 57.0 mg of 1-(3,4-methylenedioxybenzyl) piperidine in 2 mL of toluene and 2 mL of triethylamine To the solution, 12.9 mg of potassium iodide was added, stirred in the dark, and refluxed at 115° C. for 4 h. The solvent was spun off, purified through a 200-300 mesh silica gel column, and ethyl acetate:petroleum ether:triethylamine=5:20:1 (volume ratio) was used as eluent to obtain 38.0 mg of orange-red liquid (yield 46.1%) ), the compound Re-6-W. The product was analyzed by nuclear magnetic resonance, the result: 1 H NMR (400MHz, CDCl 3 )δ: 6.85(s, 1H), 6.74(s, 2H), 5.98(t, J=2.2Hz, 2H), 5.94(s, 2H), 5.40(t, J=2.2Hz, 2H), 3.41( s, 2H), 2.59(t, J=7.3Hz, 2H), 2.46(s, 8H), 2.34(t, J=7.3Hz, 2H), 1.73-1.66(m, 2H), 1.56-1.48(m , 2H), 1.37-1.29 (m, 2H). 13 C NMR (100MHz, CDCl 3 )δ:...
Embodiment 3
[0045] Example 3 Technetium-99m labeled σ 1 Synthesis of Tricarbonylcyclopentadiene Ligand Compound (Tc-4-W) of Receptor
[0046] Include the following steps:
[0047] (1) Synthesis of the labeled precursor Fe-4-W: 300.0 mg of 4-bromobutyryl ferrocene and 56.2 mg of 1-(3,4-methylenedioxybenzyl)piperidine were dissolved in 4 mL of toluene and 4 mL of Add 19.7 mg of potassium iodide to the triethylamine solution, stir in the dark, and reflux at 115°C for 4 hours. The solvent was spun off, separated and purified through a 200-300 mesh silica gel column, and petroleum ether: triethylamine=10:1 (volume ratio) was used as the eluent to obtain 97.5 mg of an orange-red solid (yield 74.1%), namely the compound Fe -4-W. The structure of the product was confirmed by nuclear magnetic resonance analysis and mass spectrometry, the result: 1 H NMR (400MHz, CDCl 3 )δ: 6.84(s, 1H), 6.73(s, 2H), 5.93(s, 2H), 4.78(t, J=1.7Hz, 2H), 4.48(t, J=1.7Hz, 2H), 4.19( s, 5H), 3.41(s, 2H), 2.75(t, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com