PSMA inhibitor, application thereof and PSMA-targeting nuclide imaging reagent
An inhibitor and imaging technology, applied in the field of biomedicine, can solve problems such as few clinical application prospects, and achieve good PSMA targeting and affinity, high cell uptake, and simple and fast labeling methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
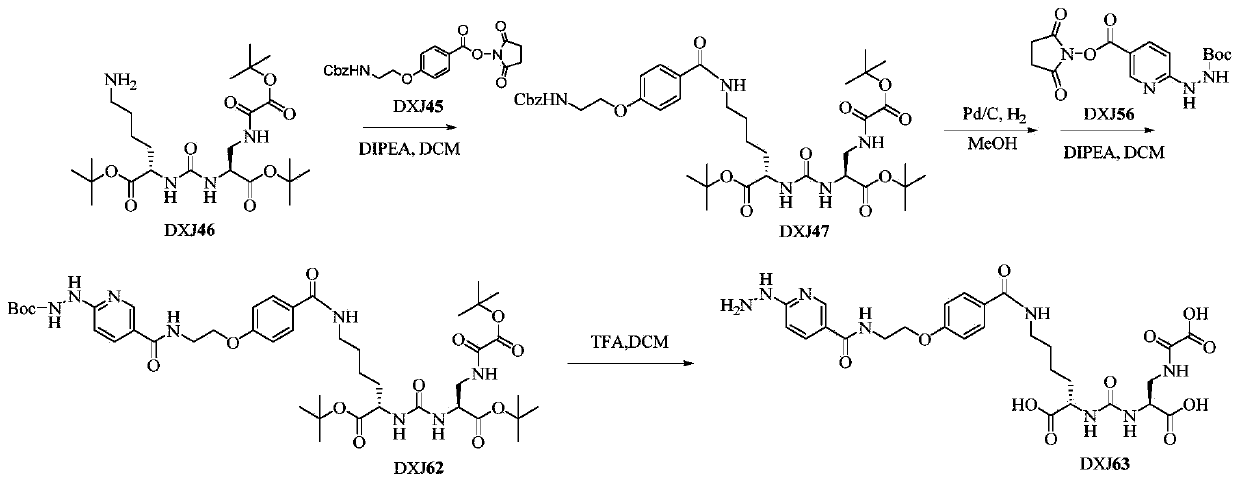
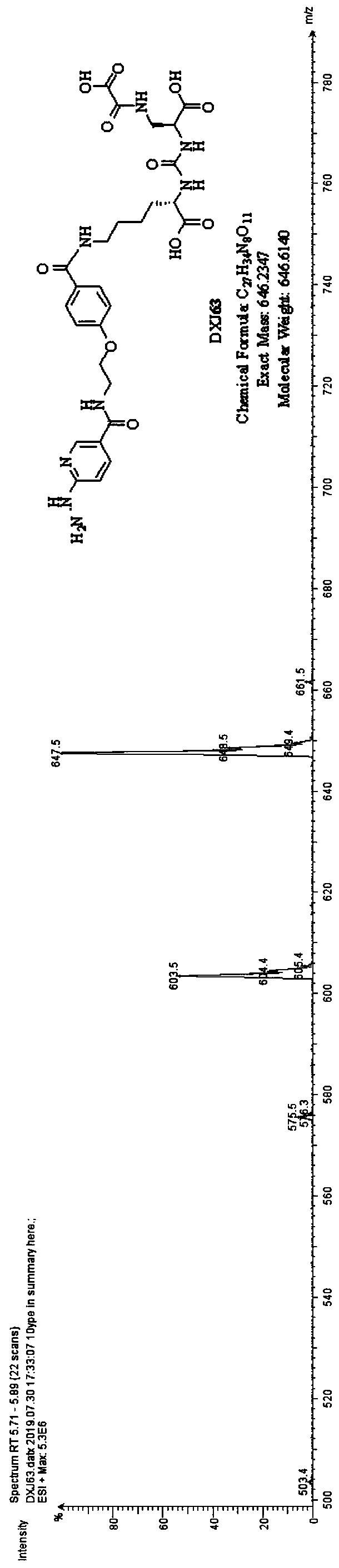
[0046] This example is used to illustrate the synthesis and characterization of monomer ODAP-PSMA-HYNIC (DXJ63), the synthetic route is as follows Picture 1-1 Shown:
[0047] DXJ46 was prepared with reference to the patent CN201910108684X authorized by this laboratory. The process is as follows: Dissolve oxalyl chloride (1.0g, 7.88mmol) in 15mL of dichloromethane, and slowly add 15mL of dichloromethane dissolved in tert-butanol (584mg, 7.88mmol) in an ice bath. Dichloromethane was reacted at room temperature under nitrogen protection for 24 hours, and the solvent was removed under reduced pressure to obtain a colorless oil. Take (s)-3-amino-2-carbonylaminopropionic acid tert-butyl ester (1.0g, 3.40mmol) and dissolve it in 20mL of dichloromethane, add triethylamine (1.38g, 13.61mmol), and add the above A colorless product (1.29g, 7.88mmol) was reacted at room temperature for 6 hours, the solvent was removed under reduced pressure, and the residue was purified by a silica gel c...
Embodiment 2
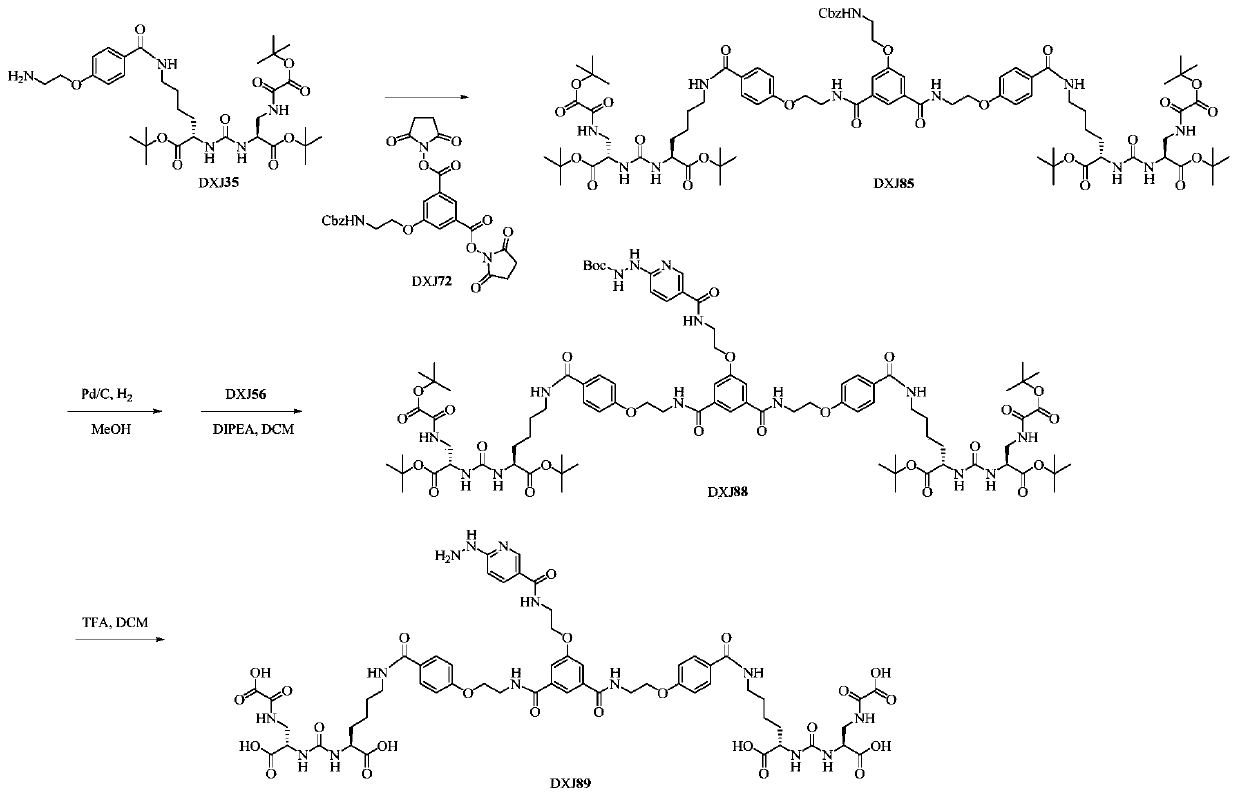
[0066] This example is used to illustrate the synthesis and characterization of monomeric GLU-PSMA-HYNIC (DXJ102), the synthetic route is as follows Figure 3-1 Shown:
[0067] DXJ49 preparation and identification reference J.Am.Chem.Soc.2014,136:18034-18043, the process is as follows: take L-glutamic acid tert-butyl ester hydrochloride (1.0g, 3.38mmol) and triethylamine (1.6 mL, 11.09mmol) was added to 30mL of dichloromethane, then cooled to -78°C, and 10mL of dichloromethane dissolved with triphosgene (341mg, 1.15mmol) was added dropwise, after the dropwise addition was completed, it was raised to room temperature, and the reaction was continued for 30 minutes; benzyloxycarbonyl-L-lysine tert-butyl ester hydrochloride (757mg, 2.03mmol) was added to the reaction solution, triethylamine (283μL, 2.03mmol) was added, and stirred overnight at room temperature; Diluted with 50mL of dichloromethane, washed twice with 100mL of water, the organic phase was dried over anhydrous sodiu...
Embodiment 3
[0083] This example is used to illustrate the ligand 99m Tc labeling and quality control.
[0084] 1. Optimization and determination of labeling conditions
[0085] The radiochemical purity of the complexes was determined using HPLC (High Performance Liquid Chromatography). There are two liquid phase conditions, System A uses water / acetonitrile / 0.1% trifluoroacetic acid as the mobile phase, System B uses ammonium phosphate buffer / acetonitrile as the mobile phase, and the gradients are shown in Table 1 and Table 2. Ammonium phosphate buffer preparation method: Take 13.61g of potassium dihydrogen phosphate, add 500mL of ultrapure water to dissolve, add 5mL of ammonia water, adjust the pH to 4.2 with phosphoric acid, add ultrapure water to dilute to 1000mL, shake well and set aside. The mobile phase needs to pass through a 0.22μm filter membrane before use, the reverse chromatographic column is Kromasil 100-5-C18, 4.6mm×250mm, and the flow rate of the mobile phase is 1.0mL / min....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com