Ortho ester derivative, liquid crystal composition, and liquid crystal display element
一种液晶组成物、群组的技术,应用在液晶性化合物,液晶性化合物及液晶组成物领域,能够解决液晶显示元件驱动电压降低、电压保持率低、介电常数各向异性不大等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0194] Hereinafter, the present invention will be described in more detail by examples, but the present invention is not limited by these examples. In addition, "%" means "wt%" unless otherwise specified.
[0195] The resulting compound is obtained by 1 The nuclear magnetic resonance spectrum obtained by the H-NMR analysis, the gas chromatogram obtained by the gas chromatography (GC) analysis, and the like are used for identification, so the analysis method will be described first.
[0196] 1 H-NMR analysis: DRX-500 (manufactured by Bruker BioSpin Co., Ltd.) was used as a measurement device. Dissolve the samples prepared in Examples etc. in CDCl 3 In the deuterated solvent in which the sample is soluble, the measurement is carried out at room temperature, 500 MHz, and the cumulative number of times is 16. In addition, in the description of the obtained nuclear magnetic resonance spectrum, s represents a singlet, d represents a doublet, t represents a triplet, q represents ...
example 1
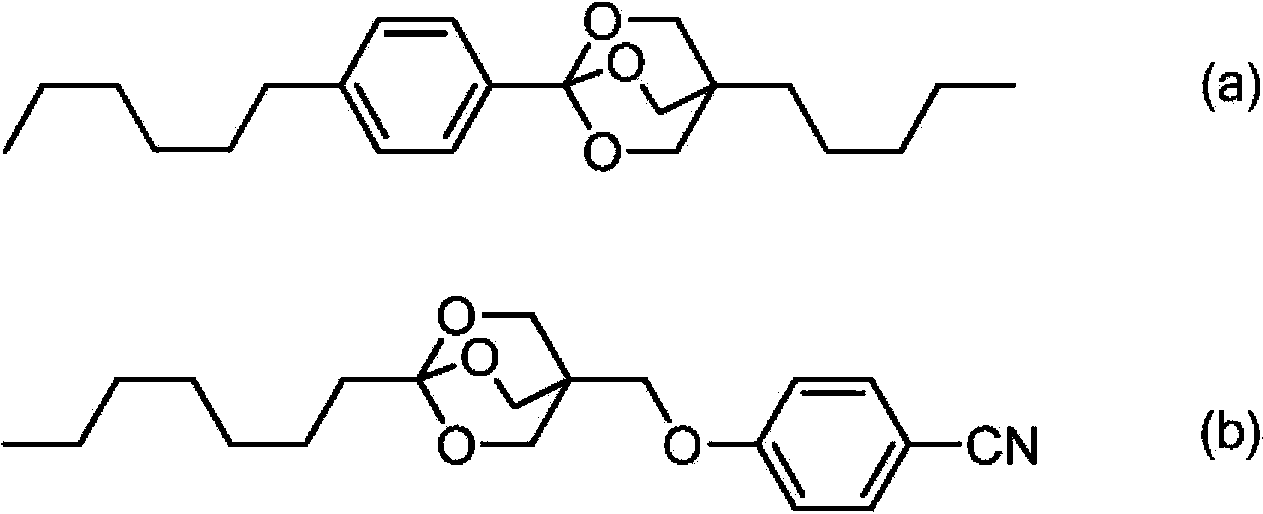
[0223] Synthesis of 4-propyl-1-(4-(3,4,5-trifluorophenyl)cyclohexyl)-2,6,7-trioxabicyclo[2.2.2]octane (No.21)
[0224]
[0225] Step 1
[0226] Add 4.8g (120mmol) of fully dry powdered sodium hydroxide, 100ml of acetonitrile and 9.9g (330mmol) of paraformaldehyde into the reactor under nitrogen atmosphere, and add 8.4g of valeraldehyde dropwise for 20 minutes while stirring at room temperature (97mmol). After the dropwise addition was completed, the temperature was 60°C. Then, the temperature was raised to 80° C. and stirred for 30 minutes. After cooling to room temperature, the insoluble matter was separated by filtration, and the solvent was distilled off under reduced pressure. The obtained residue was purified by a fractionation operation using a column chromatography (the column chromatography used ethyl acetate as a developing solvent and silica gel as a filler), and dried to obtain 2-(Hydroxymethyl)-2-propylpropane-1,3-diol 6.6 g.
[0227] Step 2
[0228] Add 2...
example 2
[0241] Synthesis of 4-ethyl-1-(4-(3,4,5-trifluorophenyl)cyclohexyl)-2,6,7-trioxabicyclo[2.2.2]octane (No.20)
[0242]
[0243] Using 3-ethyl-3-hydroxymethyl propylene oxide instead of 3-propyl-3-hydroxymethyl propylene oxide, it was synthesized in the same manner as in the third and fourth steps of Example 1, thereby synthesizing 4 -Ethyl-1-(4-(3,4,5-trifluorophenyl)cyclohexyl)-2,6,7-trioxabicyclo[2.2.2]octane.
[0244] 1 The chemical shift δ (ppm) of the H-NMR analysis is as follows, and the obtained compound can be identified as 4-ethyl-1-(4-(3,4,5-trifluorophenyl)cyclohexyl)-2,6 , 7-trioxabicyclo[2.2.2]octane. In addition, the determination solvent is CDCl 3 .
[0245] Chemical shift δ(ppm); 6.79(m, 2H), 3.91(s, 6H), 2.39(m, 1H), 1.98(m, 2H), 1.90(m, 2H), 1.60(m, 1H), 1.3 -1.1(m, 6H), 0.83(t, 3H).
[0246] The transition temperature of the obtained compound (No. 20) is shown below.
[0247] Transition temperature: C 168.5 I
[0248]Physical properties of compound...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com