Compound alpha-ketoacid tablet and preparation method thereof
A ketoacid tablet and compound technology, applied in the field of medicine, can solve problems such as unstable product production, inconvenient cleaning of instruments, complicated production process, etc., and achieve the effects of ensuring batch-to-batch stability, reducing equipment investment, and optimizing production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
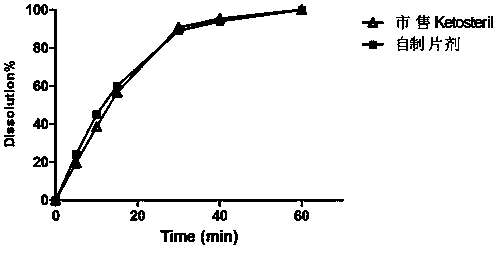
Embodiment 1
[0040] Example 1: (provided by the present invention, dry granulation, magnesium stearate is added in two proportions)
[0041]
[0042] The preparation method is:
[0043] (1) Weigh α-racemic ketoisoleucine calcium, α-ketoleucine calcium, α-ketophenylalanine calcium, α-ketovaline calcium, α-racemic hydroxymethionine Calcium, L-Lysine Acetate, L-Threonine, L-Tryptophan, L-Histidine, L-Tyrosine, Pregelatinized Starch, PVP, Stearin 0.5% by Tablet Weight Magnesium acid, through an 80 mesh sieve, is added in equal amounts and mixed evenly. Carry out dry granulation with a dry granulator, pulverize the obtained preliminary shaped tablet, pass through a 30-mesh sieve for granulation. Add the prescribed amount of polyethylene glycol, micropowder silica gel and 2.5% magnesium stearate of the tablet weight, mix well, and stamp the tablets with special shapes to obtain tablet cores;
[0044] (2) Add gastric-soluble Opadry Dissolve in 85% ethanol to prepare a coating liquid, coat...
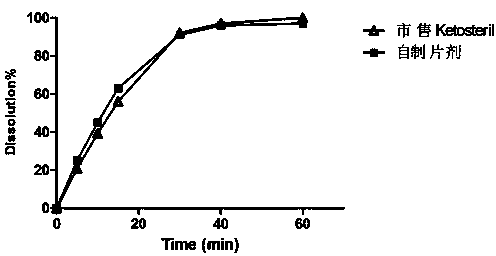
Embodiment 2
[0045] Example 2: (provided by the present invention, dry granulation, adding magnesium stearate at one time)
[0046]
[0047]
[0048] The preparation method is:
[0049] (1) Weigh α-racemic ketoisoleucine calcium, α-ketoleucine calcium, α-ketophenylalanine calcium, α-ketovaline calcium, α-racemic hydroxymethionine Calcium, L-lysine acetate, L-threonine, L-tryptophan, L-histidine, L-tyrosine, pregelatinized starch, PVP, 80 mesh sieve, equal delivery Add and mix well. Carry out dry granulation with a dry granulator, pulverize the obtained preliminary shaped tablet, pass through a 30-mesh sieve for granulation. Add the prescribed amount of polyethylene glycol, micropowder silica gel and magnesium stearate, mix well, and punch out special-shaped tablets to obtain tablet cores;
[0050] (2) Add gastric-soluble Opadry Dissolve in 85% ethanol to prepare a coating liquid, coat the tablet core in a coating pan, and dry it with air at 60°C for half an hour after coating, t...
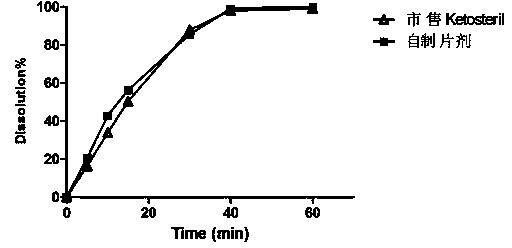
Embodiment 3
[0051] Embodiment 3: (dry granulation, no polyethylene glycol)
[0052]
[0053]
[0054] The preparation method is:
[0055] (1) Weigh α-racemic ketoisoleucine calcium, α-ketoleucine calcium, α-ketophenylalanine calcium, α-ketovaline calcium, α-racemic hydroxymethionine Calcium, L-Lysine Acetate, L-Threonine, L-Tryptophan, L-Histidine, L-Tyrosine, Pregelatinized Starch, PVP, Stearin 0.5% by Tablet Weight Magnesium acid, through an 80 mesh sieve, is added in equal amounts and mixed evenly. Carry out dry granulation with a dry granulator, pulverize the obtained preliminary shaped tablet, pass through a 30-mesh sieve for granulation. Add the prescription amount of micropowdered silica gel and 0.5% magnesium stearate of the tablet weight, mix well, and punch the tablets with special shapes to obtain tablet cores;
[0056] (2) Add gastric-soluble Opadry Dissolve in 85% ethanol to prepare a coating liquid, coat the tablet core in a coating pan, and dry it with air at 60°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com