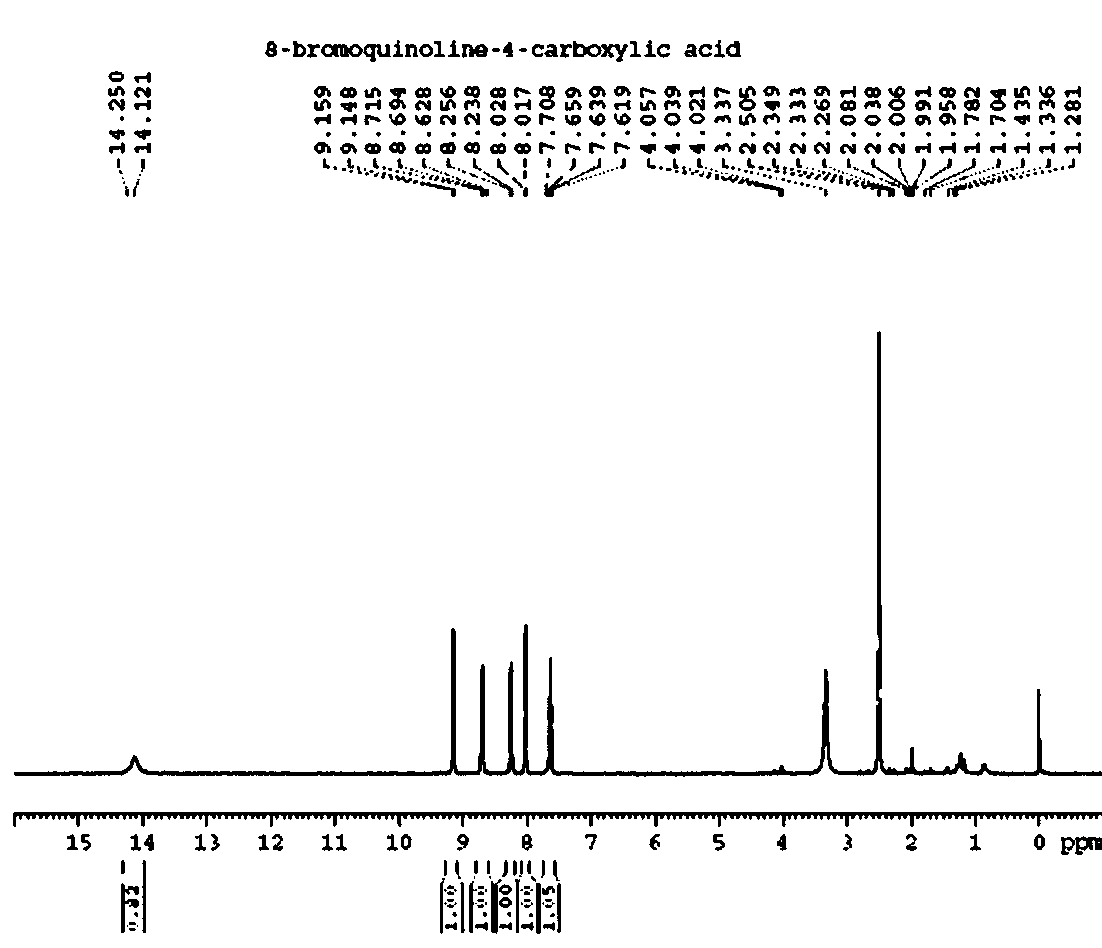
Synthesis method of 8-bromine-4-carboxyl quinoline
A technology of carboxyquinoline and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of low synthesis yield, low reaction yield, high product price and the like, and achieves high synthesis yield, high selectivity and simple synthesis steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Dissolve 50g of o-bromoaniline and 156.75g of ferric chloride hexahydrate in 750ml of glacial acetic acid, heat to 60°C, add 21.35g of butenone dropwise, and reflux (115°C) for 4 hours after the dropwise addition. Suction filter the reaction solution, wash the filter cake 3 times with dichloromethane, spin dry the filtrate, dissolve the solid obtained after spinning, adjust to alkaline with aqueous sodium hydroxide solution, then extract with dichloromethane, and combine the organic phases , dried over anhydrous sodium sulfate, filtered with suction, evaporated dichloromethane under reduced pressure, and passed the obtained crude product through a silica gel column with an eluent (petroleum ether: ethyl acetate = 40:1 V / V) to obtain a white solid 8 -Bromo-4-methylquinoline (2) 44g, yield 60%.
[0024] (2) Take 44g of the product 8-bromo-4-methylquinoline obtained in step (1) and add it to a three-necked flask, add 340ml of xylene and heat to reflux until dissolved, ...
Embodiment 2
[0027] (1) Dissolve 5g of o-bromoaniline and 11.77g of ferric trichloride hexahydrate in 75ml of glacial acetic acid, heat to 60°C, add 2.1g of butenone dropwise, and reflux (115°C) for 4 hours after the dropwise addition. Suction filter the reaction solution, wash the filter cake 3 times with dichloromethane, spin dry the filtrate, dissolve the solid obtained after spinning, adjust to alkaline with aqueous sodium hydroxide solution, then extract with dichloromethane, and combine the organic phases , dried over anhydrous sodium sulfate, filtered with suction, evaporated dichloromethane under reduced pressure, and passed the obtained crude product through a silica gel column with an eluent (petroleum ether: ethyl acetate = 40:1 V / V) to obtain a white solid 8 -Bromo-4-methylquinoline 3.74g, yield 58%.
[0028] (2) Take 3.74g of the product 8-bromo-4-methylquinoline obtained in step (1) and add it to a three-necked flask, add 35ml of xylene and heat to reflux until dissolved. Tak...
Embodiment 3
[0031] (1) Dissolve 5g of o-bromoaniline and 19.6g of ferric chloride hexahydrate in 75ml of glacial acetic acid, heat to 60°C, add 2.1g of butenone dropwise, and reflux (115°C) for 4 hours after the dropwise addition. Suction filter the reaction solution, wash the filter cake 3 times with dichloromethane, spin dry the filtrate, dissolve the solid obtained after spinning, adjust to alkaline with aqueous sodium hydroxide solution, then extract with dichloromethane, and combine the organic phases , dried over anhydrous sodium sulfate, filtered with suction, evaporated dichloromethane under reduced pressure, and passed the obtained crude product through a silica gel column with an eluent (petroleum ether: ethyl acetate = 40:1 V / V) to obtain a white solid 8 -Bromo-4-methylquinoline 3.6g, yield 56%.
[0032] (2) Add 3.6g of the product 8-bromo-4-methylquinoline obtained in step (1) into a three-necked flask, add 35ml of xylene and heat to reflux until dissolved, and take 3.6g of Se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com