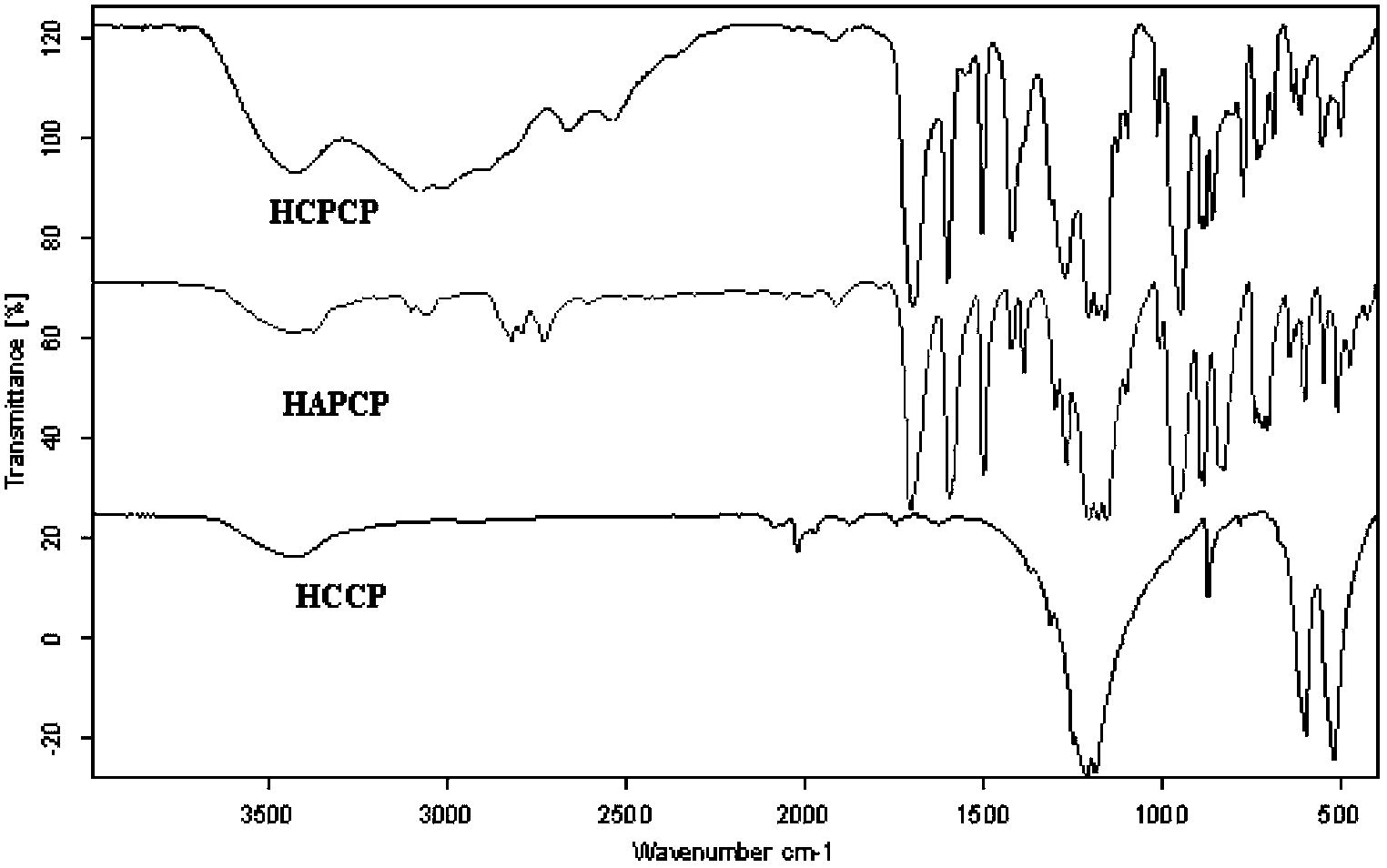
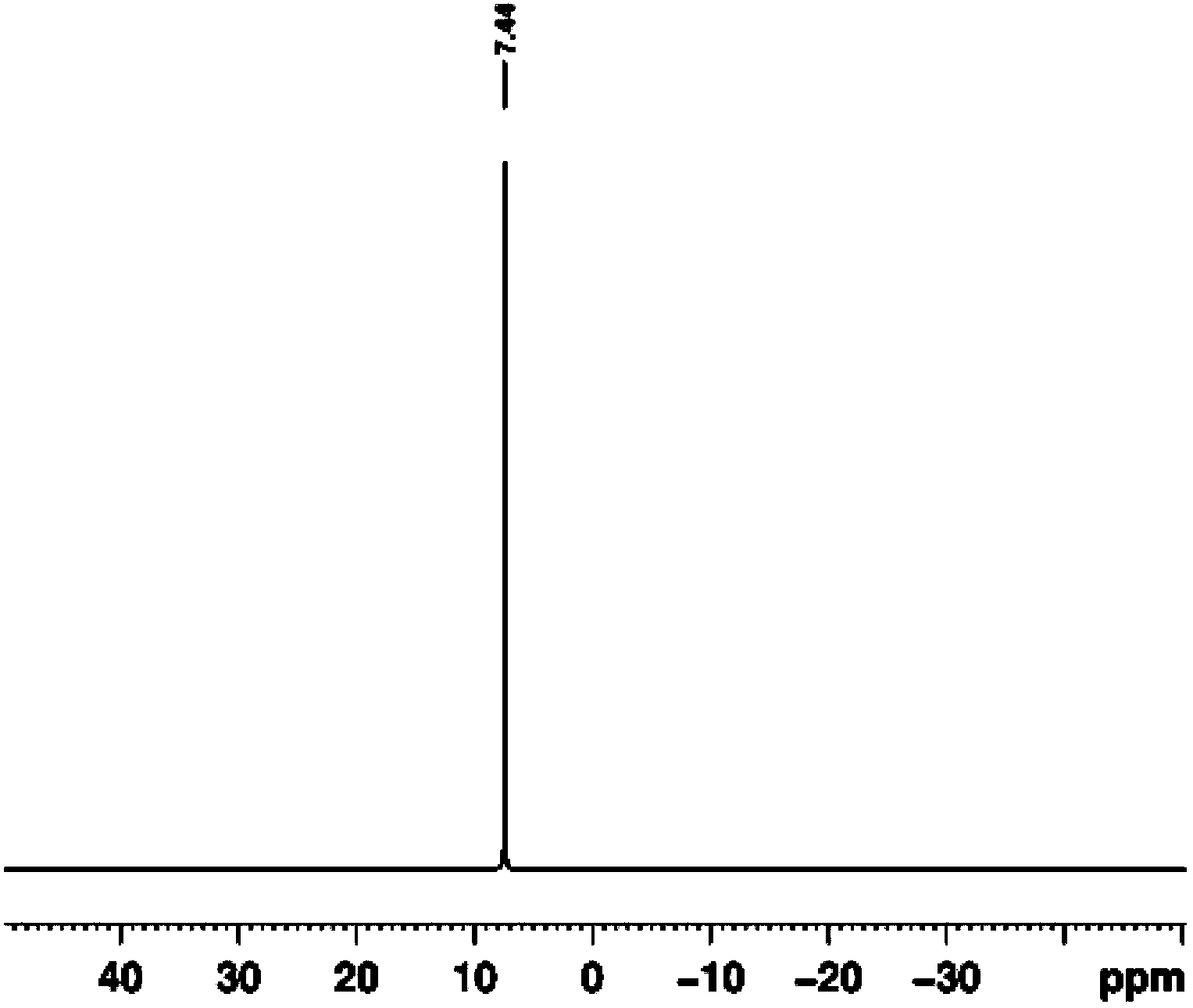
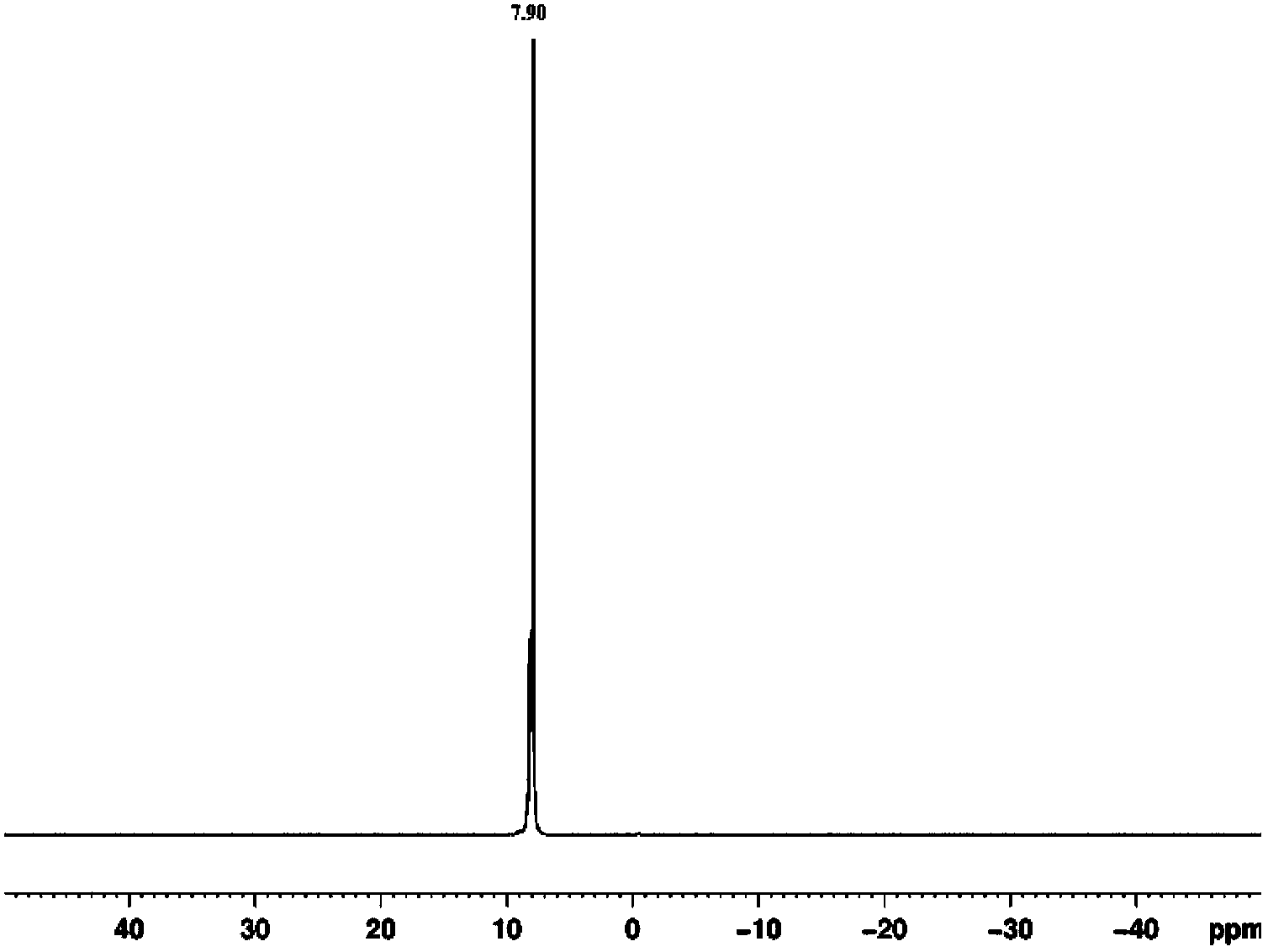
Method for preparing hexa(4-carboxylphenoxy)cyclotriphosphazene by hydrogen peroxide oxidation
A technology of cyclotriphosphazene and phenoxy, which is applied in the field of preparation of hexa-cyclotriphosphazene, can solve the problems of cumbersome post-processing, and achieve the effects of simple post-processing operation, accelerated reaction rate, and lower reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A kind of preparation method of hexa(4-carboxy-phenoxy)-cyclotriphosphazene of the present invention, adopts two-step method in essence, comprises the preparation of HAPCP and the oxidation reaction two steps of HAPCP:
[0025] (1) Preparation of intermediate product HAPCP
[0026] A. Weigh the raw materials 4-hydroxybenzaldehyde, acid-binding agent and HCCP, wherein the molar (substance amount) ratio of 4-hydroxybenzaldehyde, acid-binding agent and HCCP is 5-10:5-8:1.
[0027] B. Add an acid-binding agent (anhydrous potassium carbonate or anhydrous potassium phosphate) into the reaction vessel, then dissolve 4-hydroxybenzaldehyde in anhydrous THF, add it dropwise to the reaction vessel, stir for 0.2-2h, and The temperature of the system rises to 50-80°C, dissolve HCCP in anhydrous THF, add the anhydrous THF solution of HCCP into the constant pressure dropping funnel, add dropwise to the above reaction system at an appropriate speed, and heat and stir , After the dropw...
Embodiment 1
[0037] In a 250ml dry three-necked flask equipped with a magnet, a thermometer, a constant pressure dropping funnel, and a condensing reflux device, add crushed and dried k 2 CO 3 9.67g of powder, then dissolve 9.76g of 4-hydroxybenzaldehyde in 80ml of anhydrous THF, add it dropwise into a three-necked flask, react for 40min under stirring conditions, raise the temperature of the system to 67°C, and dissolve 3.48g of HCCP in 30ml of anhydrous THF In THF water, after the solid was completely dissolved, it was added dropwise to the reaction system for about 1 hour, and the system was refluxed at 70°C for 24 hours.
[0038] After the reaction, a white turbid solution was obtained, and the solid KCl and K were removed by suction filtration. 2 CO 3 Etc. Concentrate the filtrate to about 50ml with a rotary evaporator, pour the concentrated solution into 200ml deionized water, a white precipitate appears immediately, filter, and wash the product 3 times with deionized water repeate...
Embodiment 2
[0041] In a 500ml dry three-necked flask equipped with a magnet, a thermometer, a constant pressure dropping funnel and a condensing reflux device, add crushed and dried k 2 CO 3 16.58g of powder, then dissolve 17.08g of 4-hydroxybenzaldehyde in 130ml of anhydrous THF, add it dropwise into a three-necked flask, react for 30min under stirring conditions, raise the temperature of the system to 50°C, and dissolve 6.95g of HCCP in 50ml of anhydrous THF In THF water, after the solid was completely dissolved, it was added dropwise to the reaction system for about 1.5 hours, and the system was refluxed at 67°C for 30 hours.
[0042] After the reaction, a white turbid solution was obtained, and the solid KCl and K were removed by suction filtration. 2 CO 3 Etc. Concentrate the filtrate to about 40ml with a rotary evaporator, pour the concentrated solution into 300ml deionized water, a white precipitate appears immediately, filter, and wash the product 3 times with deionized water re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com