Benzophenone macromolecular photoinitiator and preparation method thereof
A technology of benzophenones and photoinitiators, which is applied in the field of benzophenone macromolecular photoinitiators and their preparation, can solve the problems of easy volatilization and migration, reduced initiation efficiency, poor compatibility, etc., and achieves easy Realization, the effect of simple preparation method and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
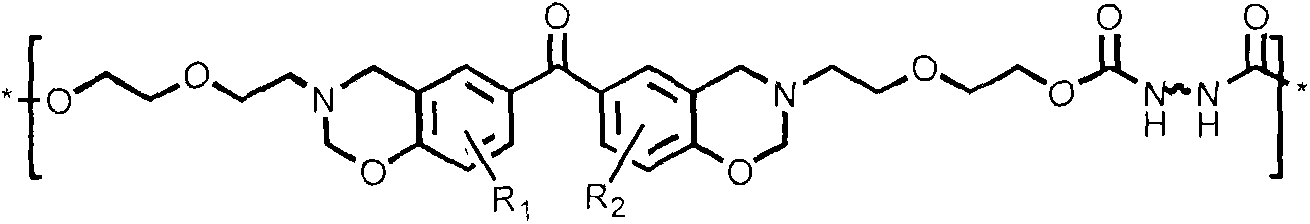
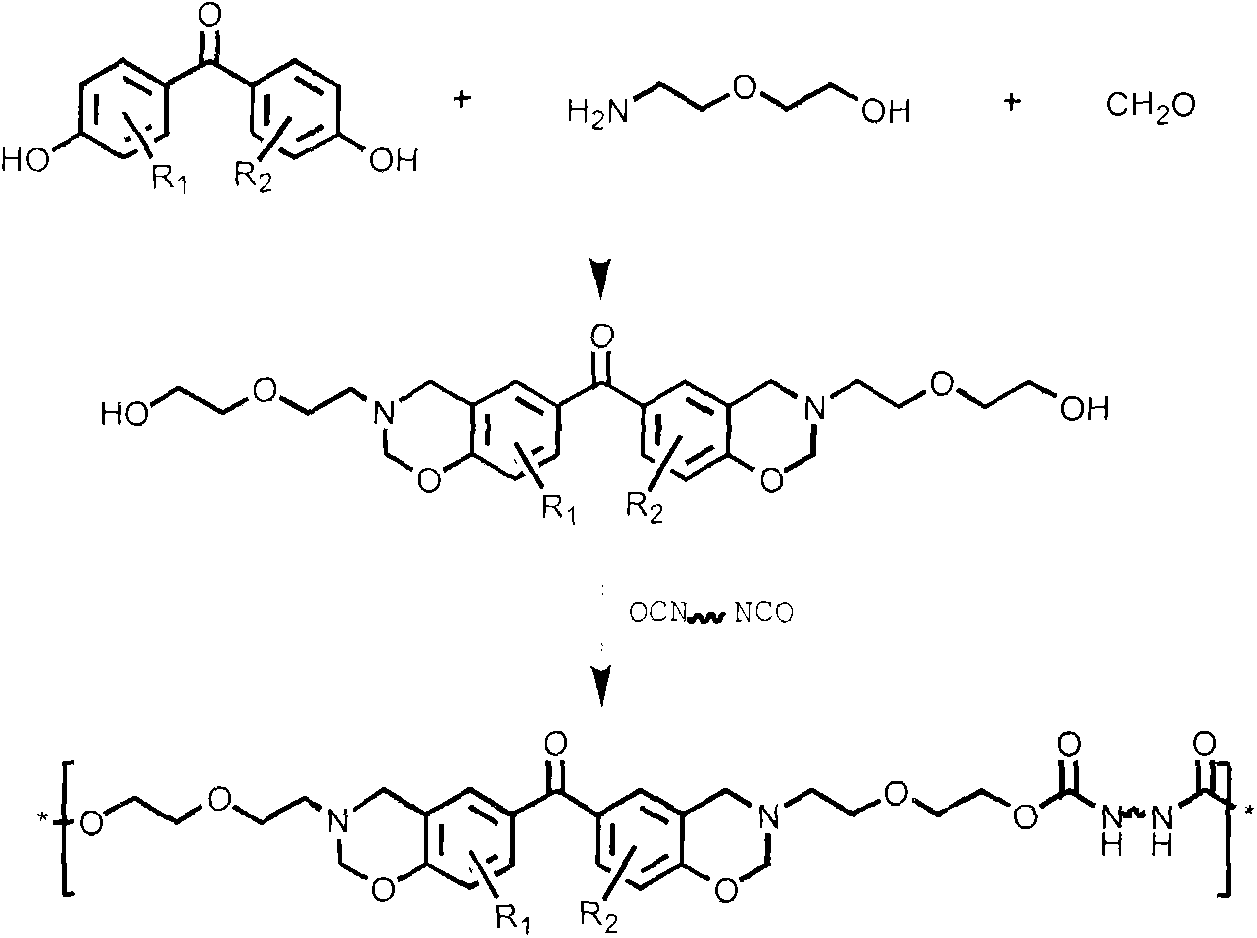
[0021] (a) Dissolve 1 part of 4.4'-dihydroxybenzophenone derivatives, 4.5 parts of paraformaldehyde, and 2 parts of diglycolamine in 50 parts of 1,4-dioxane, and stir until completely dissolve. Slowly heat to reflux at a heating rate of 5°C / min, keep the reflux reaction for 8 hours, stop heating and cool to room temperature, distill off the solvent under reduced pressure, add chloroform to dissolve, wash 5 times with 0.1N sodium hydroxide aqueous solution, deionize Wash with water 5 times. Then the organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was evaporated to remove the solvent, and the intermediate product (BZOH) was obtained after vacuum drying.
[0022] (b) Dissolve 1 part of BZOH obtained in 50 parts of dry chloroform, dissolve 0.9 parts of diisocyanate in 20 parts of dry chloroform and add it to a constant pressure dropping funnel, control the dropping speed of 4-5s / drop, and magnetically After stirring for 24 hours, the organic lay...
Embodiment 2
[0024] (a) 1 part of 4,4'-dihydroxybenzophenone derivative, 4.2 parts of paraformaldehyde, and 2 parts of diglycolamine were dissolved in 50 parts of toluene, and mechanically stirred until completely dissolved. Slowly heat to reflux at a heating rate of 5°C / min, keep the reflux reaction for 6 hours, stop heating and cool to room temperature, distill off the solvent under reduced pressure, add chloroform to dissolve, wash 5 times with 01N sodium hydroxide aqueous solution, deionized water Wash 5 times. Then the organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was evaporated to remove the solvent, and the intermediate product (BZOH) was obtained after vacuum drying.
[0025] (b) Dissolve 1 part of BZOH obtained in 50 parts of dry THF, dissolve 0.7 parts of diisocyanate in 20 parts of dry THF and add it to a constant pressure dropping funnel, control the dropping speed of 4-5s / drop, and magnetically After stirring for 24 hours, the organic layer...
Embodiment 3
[0027] (a) 1 part of 4,4'-dihydroxybenzophenone derivatives, 4.5 parts of paraformaldehyde, and 2 parts of diglycolamine were dissolved in 50 parts of xylene, and mechanically stirred until completely dissolved. Slowly heat to reflux at a heating rate of 5°C / min, keep the reflux reaction for 6 hours, stop heating and cool to room temperature, distill off the solvent under reduced pressure, add chloroform to dissolve, wash 5 times with 0.1N sodium hydroxide aqueous solution, deionize Wash with water 5 times. Then the organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was evaporated to remove the solvent, and the intermediate product (BZOH) was obtained after vacuum drying.
[0028] (b) Dissolve 1 part of BZOH obtained in 50 parts of dry DMSO, dissolve 0.8 parts of diisocyanate in 20 parts of the above-mentioned DMSO and add it to the constant pressure dropping funnel, control the dropping speed of 4-5s / drop, at room temperature After magnetically...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com