A kind of fully bio-based polyester containing double bonds and its preparation method and application
A technology of all-bio-based polyester, which is applied in the field of polyester preparation and can solve the problems of unsaturated all-bio-based polyester.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
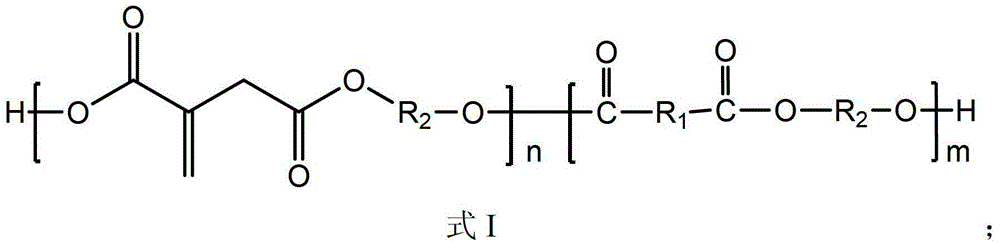
[0057] (1) Under the protection of nitrogen, 0.5mol of itaconic acid, adipic acid (HOOC-R 1 -COOH,R 1 and R in formula I 1 have the same meaning) 0.5mol, decanediol (HO-R 2 -OH, R 2 and R in formula I 2 Have the same meaning) 1.3mol, p-toluenesulfonic acid 0.001mol, triphenyl phosphate 1.86g, hydroquinone 2.33g, add into the reactor, raise the temperature of the reaction system to 150°C, and carry out the esterification reaction under the pressure of 100KPa, Fully stir during the reaction to remove the water produced by the reaction, and the reaction time is 1 h.
[0058] (2) After the esterification reaction, raise the temperature of the reaction system to 200°C, and gradually reduce the pressure to 50KPa, react for 0.5h, then continue to vacuumize to about 96Pa, continue to react for 3h, and discharge the material after the reaction to obtain the product (full bio-based polyester with double bonds).
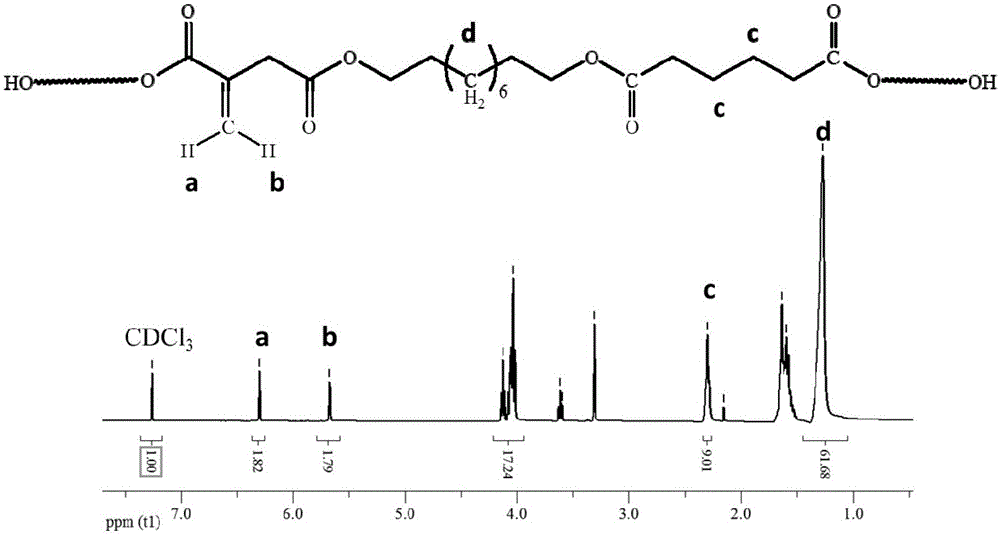
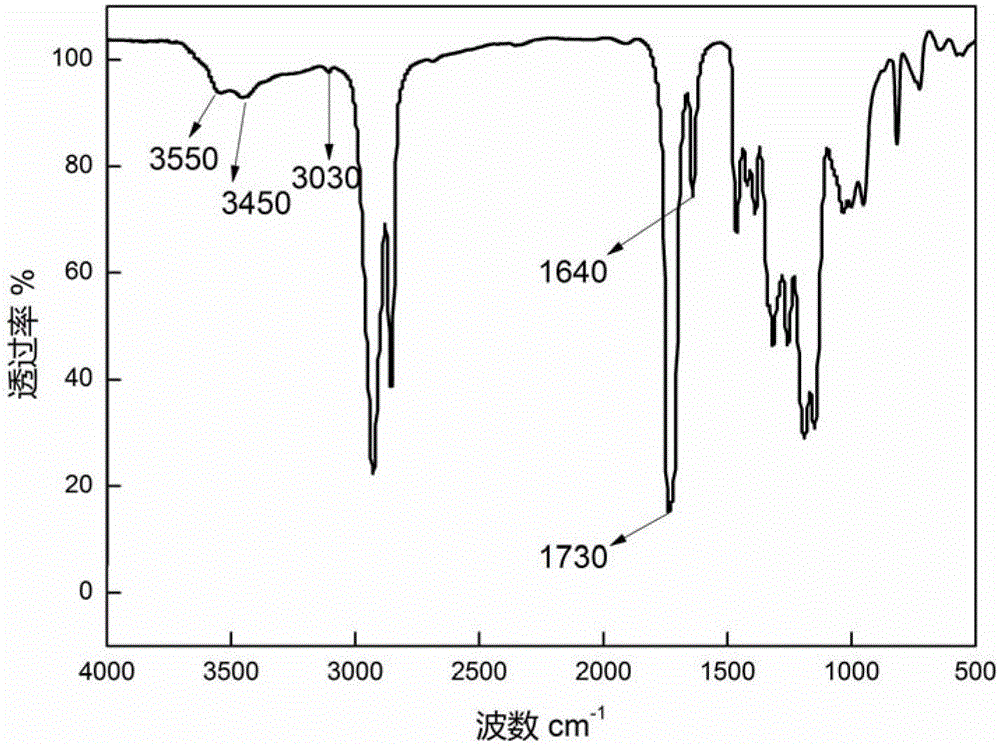
[0059] The proton nuclear magnetic resonance spectrum of the fully b...
Embodiment 2
[0066] (1) Under the protection of nitrogen, 0.5mol of itaconic acid, adipic acid (HOOC-R 1 -COOH,R 1 and R in formula I 1 have the same meaning) 0.5mol, decanediol (HO-R 2 -OH, R 2 and R in formula I 2 Have the same meaning) 1.8mol, 0.01mol of p-toluenesulfonic acid, 0.466g of triphenyl phosphate, and 0.05g of hydroquinone are added to the reactor, the temperature of the reaction system is raised to 180°C, and the esterification reaction is carried out at a pressure of 20KPa. Fully stir during the reaction to remove the water produced by the reaction, and the reaction time is 2.5h.
[0067] (2) After the esterification reaction, raise the temperature of the reaction system to 220°C, and gradually reduce the pressure to 20KPa, react for 1h, then continue to vacuumize to about 96Pa, continue to react for 2h, and discharge the material after the reaction to obtain the product ( fully bio-based polyester with double bonds).
[0068] According to the proton nuclear magnetic ...
Embodiment 3
[0075] (1) Dimethyl itaconate 1mol, 1,4-butanediol (HO-R 2 -OH) 0.5mol, dodecanediol (HO-R 2 -OH) 0.5mol into the reactor protected by inert gas, then raise the temperature of the reaction system to 150°C, react for 0.5h, then raise the temperature to 180°C, keep the temperature at this temperature for 1h, carry out transesterification reaction at normal pressure until methanol flows out The amount reaches about 95wt% of the theoretical value.
[0076] Among them, R in 1,4-butanediol 2 for R in dodecanediol 2 for
[0077] (2) Lower the temperature of the reaction system to 150°C, connect to the vacuum system, evacuate to 50KPa, and react for 1 hour to remove unreacted monomers and small molecule by-products in the reaction system. Stop vacuuming and feed nitrogen, add 0.005 mol of tetrabutyl titanate, 0.3 g of triphenyl phosphate, 10100.5 g of antioxidant and make them completely dispersed, stop nitrogen, continue vacuuming to below 100 Pa, and raise the temperature to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com