Method for preparing shape-controlled alpha type iron trioxide micro/nano material
A ferric oxide, micro-nano technology, applied in the fields of iron oxide, iron oxide/iron hydroxide, nanotechnology for materials and surface science, etc., to achieve good repeatability, high crystallinity, and controllable morphology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 The preparation method of spherical α-type iron trioxide includes the following steps:
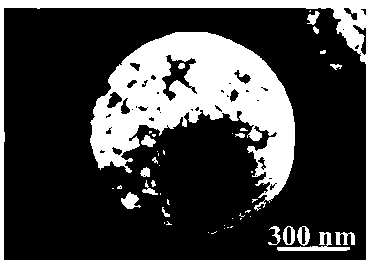
[0023] Take 8mL of 200mmol / L ferric chloride aqueous solution, add 32mL of 50mmol / L HEPES aqueous solution, stir well, transfer to a stainless steel reactor lined with polytetrafluoroethylene, seal it and put it in a constant temperature oven at 150℃ to react for 6h, then take out the reaction The kettle is naturally cooled in the air. The red precipitate in the kettle is separated by centrifugation, and the precipitate is washed with absolute ethanol and deionized water. Finally, it is dried in an oven at 60°C for 12 hours to obtain a diameter of Spherical α-type iron trioxide of 200~900nm.
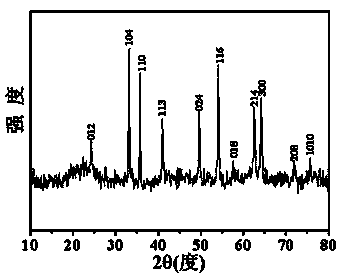
[0024] Attached figure 1 It is the XRD spectrum of spherical α-type ferric oxide. It can be seen from the XRD spectrum that the XRD spectrum of the obtained sample is consistent with the standard pattern of α-type ferric oxide (JCPDS No. 33-0664), indicating the preparation The result is...
Embodiment 2
[0025] Embodiment 2 The preparation method of spherical α-type iron trioxide includes the following steps:
[0026] Take 8mL of 200mmol / L ferric chloride aqueous solution, add 16mL of 100mmol / L HEPES aqueous solution, stir well, transfer to a stainless steel reactor lined with polytetrafluoroethylene, seal it and put it in a 160℃ constant temperature oven to react for 12h, then take out the reactor. Naturally cool in the air, separate the red precipitate in the kettle by centrifugation, and wash the precipitate with absolute ethanol and deionized water, and finally dry it in an oven at 60°C for 12 hours to obtain a diameter of 700nm~ 1.8μm spherical α-type iron trioxide.
Embodiment 3
[0027] Example 3 The preparation method of porous rod-shaped α-type iron trioxide includes the following steps:
[0028] Take 8mL of 200mmol / L ferric chloride aqueous solution, 8mL of 200mmol / L ferrous sulfate aqueous solution and 16mL of 100mmol / L HEPES aqueous solution, stir well, transfer to a stainless steel reactor lined with polytetrafluoroethylene, seal and put into React in a constant temperature oven at 180°C for 12 hours, then take out the reaction kettle and let it cool naturally in the air. The yellow precipitate in the kettle is separated by centrifugation, and the precipitate is washed with absolute ethanol and deionized water, and finally in a muffle furnace Heat treatment at 300°C for 2h, take it out and cool it in a desiccator to obtain a porous rod-shaped α-type iron trioxide with a length of 50-100nm and a diameter of 10-20nm.
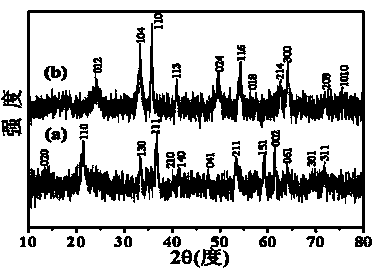
[0029] Attached image 3 It is the XRD spectrum of the prepared porous rod-shaped α-type iron trioxide. It can be seen from the XRD spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com