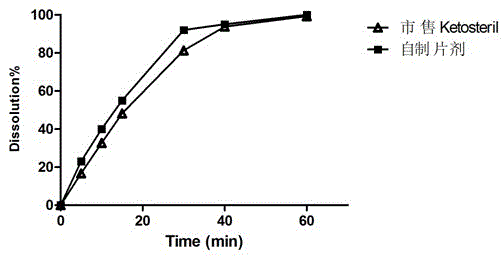
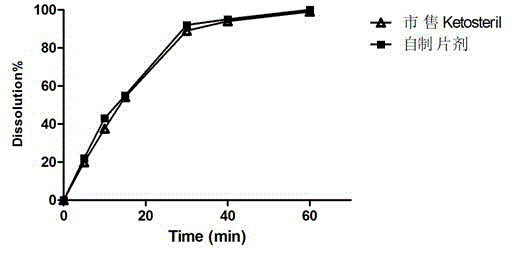
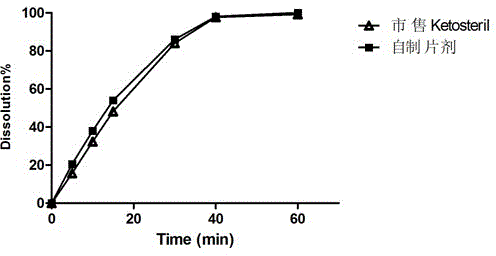
A kind of talc-free compound alpha-keto acid tablet and its preparation process
A technology of ketoacid tablets and talc powder, which is applied in the field of medicine, can solve the problems affecting inter-batch variability, small quality, poor quality uniformity, etc., and achieves the effects of ensuring inter-batch stability, preventing interaction and ensuring stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Below in conjunction with embodiment do further explanation. But this is only an example, and the content of the present invention is not limited thereto. Embodiment 1: (provided by the present invention, separate wet granulation)
[0053] Alkaline components:
[0054]
[0055]
[0056] Slightly acidic and near-neutral components:
[0057]
[0058] Extras:
[0059]
[0060] The preparation method is:
[0061] (1) Preparation of alkaline part
[0062] Weigh α-racemic ketoisoleucine calcium, α-ketoleucine calcium, α-ketophenylalanine calcium, α-ketovaline calcium, α-racemic hydroxymethionine calcium, L - Histidine, PVPP, equal amount and mix evenly; weigh PVP, dissolve in appropriate amount of alcohol solution, prepare a binder with a concentration of 5%-15% (g / ml), add it to the mixed alkaline The main drug is made of soft materials, granulated, dried, granulated, and the alkaline part is obtained;
[0063] (2) Preparation of acidic and near-neutral pa...
Embodiment 2
[0067] Embodiment 2: (separate wet granulation, the solvent of binder is aqueous solution)
[0068] Alkaline components:
[0069]
[0070] Slightly acidic and near-neutral components:
[0071]
[0072] Extras:
[0073]
[0074] The preparation method is:
[0075] (1) Preparation of alkaline part
[0076] Weigh α-racemic ketoisoleucine calcium, α-ketoleucine calcium, α-ketophenylalanine calcium, α-ketovaline calcium, α-racemic hydroxymethionine calcium, L - Histidine, PVPP, equal amount and mix evenly; weigh PVP, dissolve in appropriate amount of aqueous solution, prepare a binder with a concentration of 5%-15% (g / ml), add it to the mixed alkaline The main drug is made of soft materials, granulated, dried, and granulated to obtain the alkaline part;
[0077] (2) Preparation of acidic and near-neutral parts
[0078] Weigh the prescribed amount of L-lysine acetate, L-threonine, L-tryptophan, L-tyrosine, pregelatinized starch, PVPP, and mix evenly in equal amounts; ...
Embodiment 3
[0080] Example 3: (separate wet granulation, only internally added disintegrant)
[0081] Alkaline components:
[0082]
[0083] Slightly acidic and near-neutral components:
[0084]
[0085] Extras:
[0086] Polyethylene glycol 10.8mg
[0087] Micronized silica gel 5.4mg
[0088] Magnesium Stearate 15mg
[0089] The preparation method is:
[0090] (1) Preparation of alkaline part
[0091] Weigh α-racemic ketoisoleucine calcium, α-ketoleucine calcium, α-ketophenylalanine calcium, α-ketovaline calcium, α-racemic hydroxymethionine calcium, L - Histidine, PVPP, equal amount and mix evenly; weigh PVP, dissolve in appropriate amount of alcohol solution, prepare a binder with a concentration of 5%-15% (g / ml), add it to the mixed alkaline The main drug is made of soft materials, granulated, dried, granulated, and the alkaline part is obtained;
[0092] (2) Preparation of acidic and near-neutral parts
[0093] Weigh the prescribed amount of L-lysine acetate, L-threonine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com