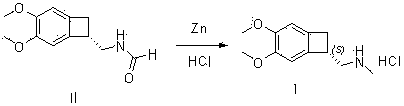Method for synthesizing ivabradine key intermediate
A technology of ivabradine and its synthesis method, which is applied in the field of drug synthesis, and can solve the problems of cumbersome post-processing, unfriendly environment, and severe reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
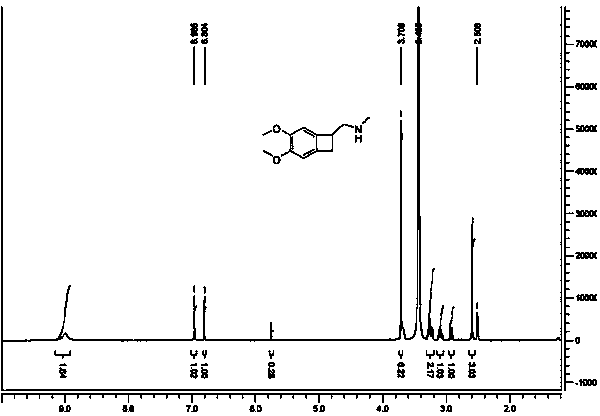
[0046]96.5g (0.5mol) of 4,5-dimethoxy-1-aminomethyl-benzocyclobutane was heated and dissolved in 500ml of ethanol, and 94.5g (0.5mol) of N-acetyl-L-glutamic acid was added , after the reaction is finished, cool to about 20°C, let it stand for 12 hours, crystals are precipitated, and the salt of formula (III) is obtained by suction filtration.
[0047] Dissolve the above salt in 200ml of pure water, adjust the pH to 10 with 16g (0.4mol) of 40% sodium hydroxide, add 200mol*3 dichloromethane to extract 3 times, combine the organic layer, add 50g of anhydrous sodium sulfate to dry 6-8 Hours, the desiccant was filtered off, and the solvent was spin-dried in vacuo to obtain a yellow oil, 33.78g, with a yield of 70%.
Embodiment 2
[0049] 96.5g (0.5mol) of 4,5-dimethoxy-1-aminomethyl-benzocyclobutane was heated and dissolved in 500ml of methanol, and 94.5g (0.5mol) of N-acetyl-L-glutamic acid was added , after the reaction was completed, cooled to about 20°C, stood still for 12 hours, crystals were precipitated, and filtered with suction to obtain the salt of (S)-4,5-dimethoxy-1-aminomethyl-benzocyclobutane, such as Salts of formula (III).
[0050] Dissolve the above salt in 200ml of pure water, adjust the pH to 10 with 16g (0.4mol) of 40% sodium hydroxide, add 200mol*3 dichloromethane to extract 3 times, combine the organic layer, add 50g of anhydrous sodium sulfate to dry 6-8 hour, filter off the desiccant, and spin dry the solvent in vacuo to obtain a yellow oil, i.e. (S)-4,5-dimethoxy-1-aminomethyl-benzocyclobutane, 30.45g, yield 63.21% .
Embodiment 3
[0052] Formula (III) compound (S)-4,5-dimethoxy-1-aminomethyl-benzocyclobutane, 33.78g, dissolved in 100ml formic acid, added ZnCl 2 , 5g, the reaction temperature is about 65°C, stir the reaction for 5 hours, after the reaction, the temperature of the reaction solution is lowered to below zero, add 500ml of water dropwise, add 300ml of dichloromethane*2 for extraction twice, wash once with 100ml of saturated sodium chloride solution, 200ml *2 Washed twice with water, dried with anhydrous sodium sulfate and then spin-dried to obtain (S)-4,5-dimethoxy-1-formamidomethyl-benzocyclobutane, such as formula (II), shallow Yellow oil, 33.65g, yield 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com