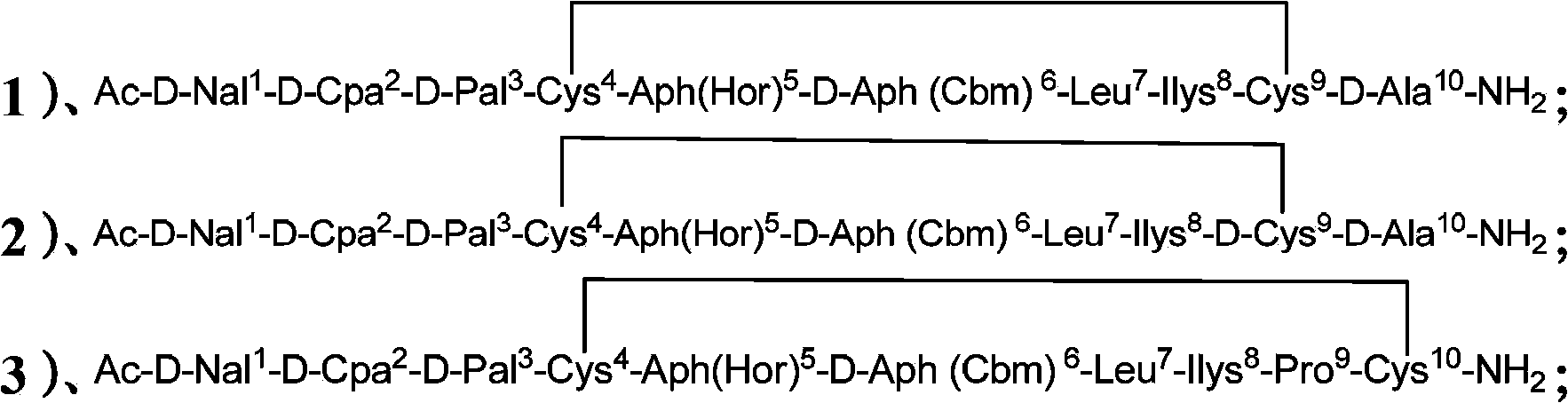
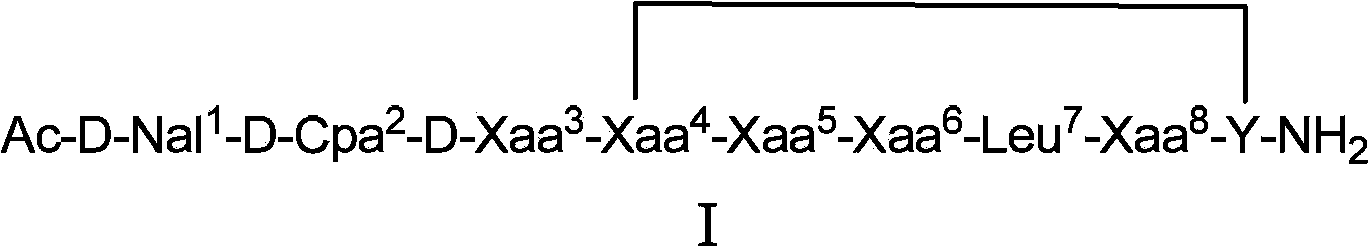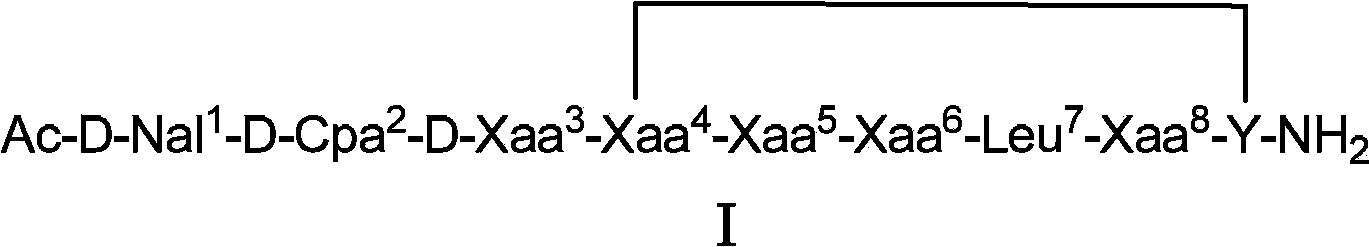Cyclopeptide luteinizing hormone releasing hormone (LHRH) antagonist derivatives and pharmaceutical use thereof
A use and physiological technology, applied in the field of secreting steroids, can solve the problems of LHRH antagonist drugs that are difficult to be absorbed orally, high histamine release, and low bioavailability, and achieve good antagonistic activity, high enzyme stability, and high enzyme activity. Effects of stability and lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Synthesis of Cyclic Peptide LHRH Antagonist Derivatives
[0079] Weigh 0.5g of MBHA resin and place it in the reactor, add DCM (4-dicyanomethylene) to swell for 30min, and stir magnetically to disperse the resin evenly. Drain, wash with DCM, methanol (MeOH), DCM (3×2min) (the same below), and drain. Add 10% DIEA / DCM to neutralize (2×5min), release the amino group, wash the resin, and drain it. (1) Add 1.08mmol of Fmoc-D-Ala-OH, 1.08mmol of HOBt, 1.08mmol of DIC (N,N'-diisopropylcarbodiimide) in 2mL of CH 3 OH, 4mL DCM at room temperature for 4h. (2) Wash, drain, and take a little resin to detect with ninhydrin indicator. Take a little resin in a small glass test tube, and add two drops of each of the three solutions of ninhydrin in sequence. Heat at 110°C for 5min. Positive: Most of the resin is blue or light red, and the solution is blue; Negative: The resin is colorless and transparent, and the solution is light yellow. If positive, repeat 1. (3) If ...
Embodiment 2
[0087] Embodiment 2: In vivo testosterone inhibition experiment in rats
[0088] The body weight of the animals (SD male rats) was weighed before the experiment, and the blood was collected from the venous plexus behind the glass capillary bulb. 8, 16, and 24 hours after excessive subcutaneous injection or intragastric administration of the compound to be tested (different doses), blood was collected from the retrobulbar venous plexus, centrifuged at 5000 rpm for 8 minutes, and the separated serum was analyzed by chemiluminescence (Access Immunoassay System, Beckman Coulter, USA). Luminometer) to measure serum testosterone levels. Please refer to Table 2 for specific results. It can be seen that the compound of the present invention can effectively inhibit the activity of testosterone in rats.
Embodiment 3
[0089] Example 3: Determination of Antagonistic Activity on GnRH (Gonadotropin-releasing Hormone) Receptor
[0090] The calcium ion-sensitive fluorescent dye is used to detect the function of GnRH receptor. When the GnRH receptor is activated by the agonist, it can trigger the release of calcium ion in the endoplasmic reticulum. The calcium ion can combine with the fluorescent dye to increase the fluorescence intensity. The stronger the fluorescence signal, the stronger the activity of the ligand or agonist, otherwise the weaker the activity of the ligand or agonist. Since the antagonistic compounds can competitively bind to the GnRH receptor, the release of calcium ions in the endoplasmic reticulum is reduced, and the fluorescence signal is also reduced. The inhibitory activity of the compound to be tested on the GnRH receptor is characterized according to the degree of signal reduction.
[0091] CHO-K1 / Gα15 cells (purchased from GenScript) stably expressing GnRH receptors w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com