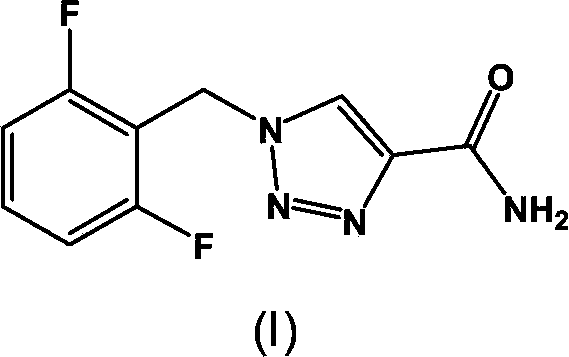
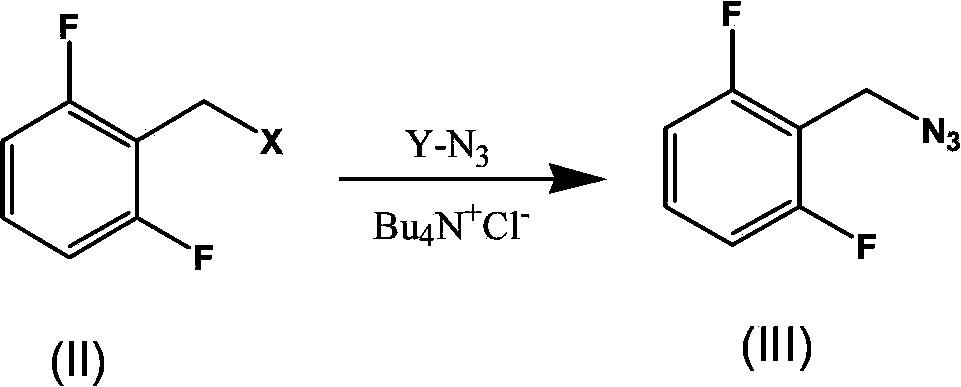
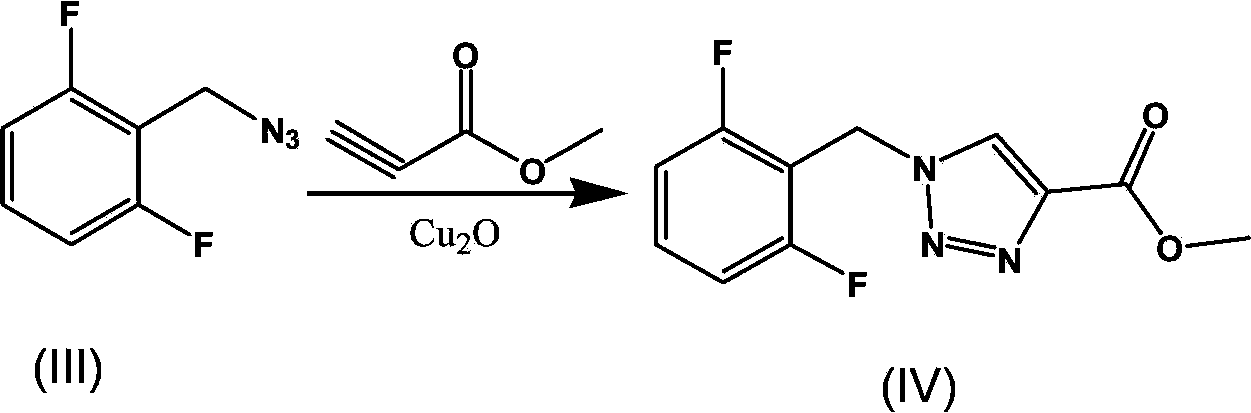
Synthesis process of rufinamide
A rufinamide and process technology, applied in the field of medicinal chemistry, can solve the problems of long reaction time, low catalytic efficiency, complicated operation and the like, and achieve the effects of reducing reaction time, improving catalytic efficiency and speeding up the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of 1 nanometer cuprous oxide catalyst
[0026] In a 1000ml three-necked bottle, add 1.6g PVP, 0.44g copper acetate, and 200ml water, and slowly add 40ml of sodium borohydride solution containing 0.75g of sodium borohydride into the copper acetate solution under vigorous stirring. After that, the vigorous stirring was continued for 2 h. Centrifuge the reaction solution in the previous step (6000rpm) to discard the supernatant, add water to wash, and centrifuge again (6000rpm) to obtain about 2g of a black solid, wherein the copper content is about 60%.
Embodiment 1
[0027] The preparation of embodiment 1 rufinamide
[0028] Add 2.88g of sodium azide, 0.9g of tetrabutylammonium chloride, and 10ml of water into a 100ml three-necked flask, stir at room temperature, and add 20ml of acetonitrile containing 3g of 2,6-difluorobenzyl bromide after all the solids are dissolved solution. The mixture was stirred at room temperature and monitored by TLC (petroleum ether:ethyl acetate=30:1). The reaction takes about 2 hours to complete. Stirring was stopped, the layers were allowed to stand, and the acetonitrile layer was separated to obtain an acetonitrile solution of 2-(azidomethyl)-1,3-difluorobenzene. Under the protection of nitrogen, add 1.4ml methyl propiolate and nano Cu to the acetonitrile solution of 2-(azidomethyl)-1,3-difluorobenzene 2 O (0.01 equivalent, 0.034 g), and the reaction was stirred at room temperature under nitrogen protection, and monitored by TLC (dichloromethane:methanol=30:1). The reaction takes about 3 hours to complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com