Long-acting recombinant follicle-stimulating hormone and application thereof
A fusion protein, hfsh-fc technology, applied to long-acting recombinant human follicle-stimulating hormone fusion protein, its preparation method and application field, can solve the problems of low expression of recombinant hFSH, complicated preparation process, frequent injection and administration, etc. The effect of prolonging the half-life in vivo, improving protein activity and reducing the number of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1. Construction of gene expression vector encoding recombinant hFSH-Fc fusion protein
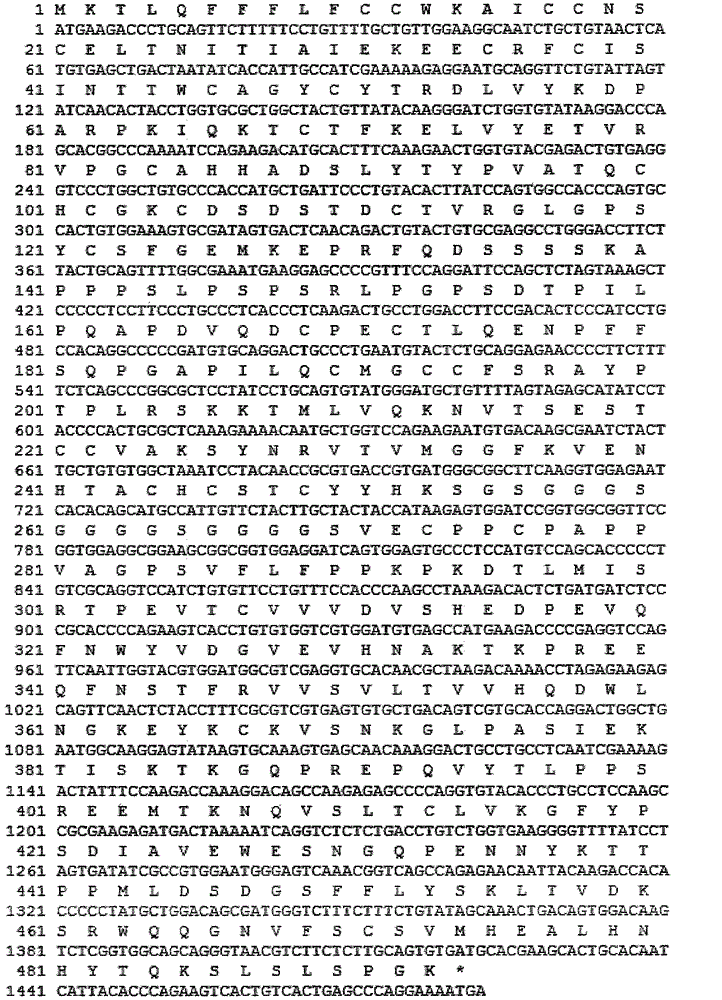
[0062] The gene sequence design was optimized based on the preferred codons of CHO cells, and the optimized fusion gene containing the signal peptide encoding hFSH protein β chain and its mature peptide, CTP and hFSH α chain mature peptide was synthesized by artificial synthesis. The synthetic 756bp DNA fragment was inserted between the EcoRV restriction sites in the transfer vector such as pUC57 to obtain the hFSH plasmid (phFSH), and the correctness of the inserted sequence was verified by DNA sequencing.
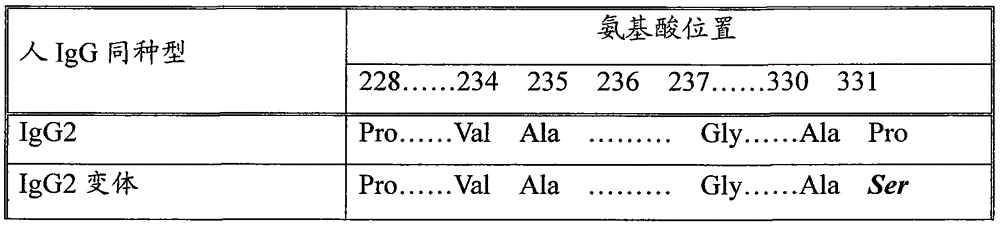
[0063] The fusion gene L-vIgG2Fc encoding the flexible peptide linker (Linker, detect "L") and the Fc variant (vIgG2Fc) fragment containing BamHI (5' end) and EcoRI (3' end) restriction sites were artificially synthesized respectively. The obtained fusion gene fragments were respectively inserted into transfer vectors such as PUC19 between the BamHI and EcoRI sites to ob...
Embodiment 2
[0066] Example 2. Stable expression of recombinant hFSH-Fc fusion protein in mammalian cells
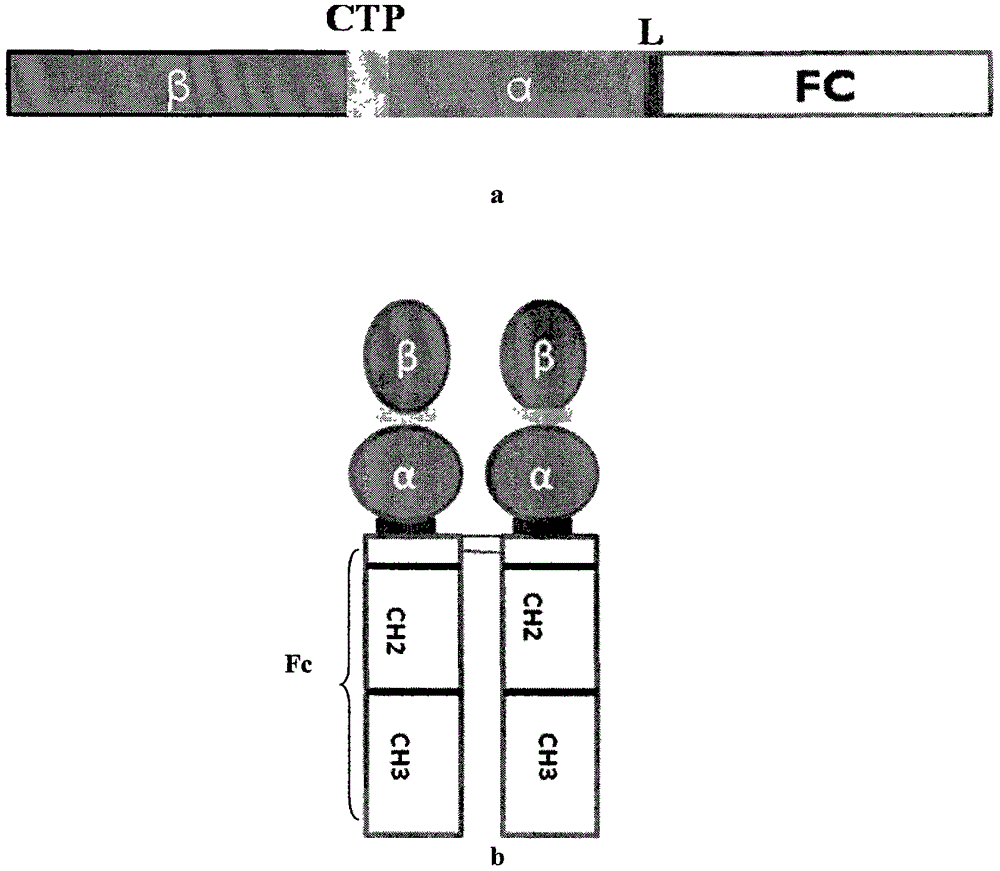
[0067] The expression plasmid pCDNA3-hFSH-L-Fc constructed in Example 1 was transfected into DHFR enzyme-deficient CHO host cells (CHO-DHFR - ), figure 2 b shows a schematic diagram of the recombinant dimerized hFSH-Fc fusion protein. Transfection was carried out by electroporation, using a Gene Pulser Electroporator (Bio-Rad Laboratories, Hercules, CA) with a capacitance of 960 μF, setting its electric field to 250 V, and 2 to 5 × 10 cells in the cuvette. 7 Add 10 μg of plasmid DNA linearized with PvuI to each cell. Two days after transfection, the medium was changed to a growth medium containing 100 μg / mL Zeocin resistance marker gene to obtain transfectants that had passed the initial resistance screening. Using the method of western blotting, use anti-hFSH antibody to detect the expression of hFSH-Fc, such as Figure 6 . The use of DHFR to amplify the selectable marker gene...
Embodiment 3
[0068] Example 3. Production and purification of recombinant hFSH-Fc fusion protein
[0069] The high-yield cell strain obtained in Example 2 was first acclimatized in a culture dish without serum, and then transferred to a shake flask for suspension acclimatization. During the acclimatization process, the medium was screened at the same time, and different components were added to observe the growth of the cells. Growth state, growth trend, and biochemical indicators such as the activity of the expressed product and sialic acid, the preferred cell culture conditions are: adding 100 μM Cu2+ to the basal medium, adding 2 mM Man NAc (N-acetyl-D-aminomannose) to the feeding medium ), the method can increase the glycosylation degree of the recombinant hFSH-Fc fusion protein, and increase the sialic acid content by about 20%. After the acclimatization is successful, the cells are expanded to a sufficient amount, and the 7L bioreactor is monitored for culture. When the cell density ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com