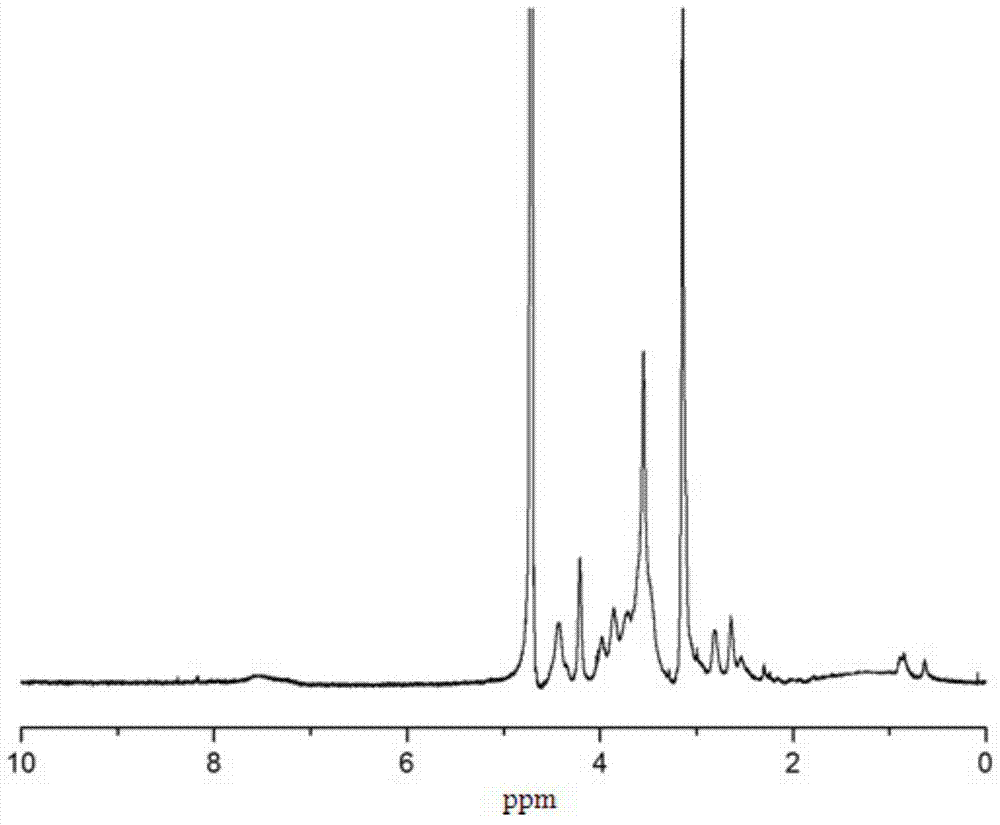
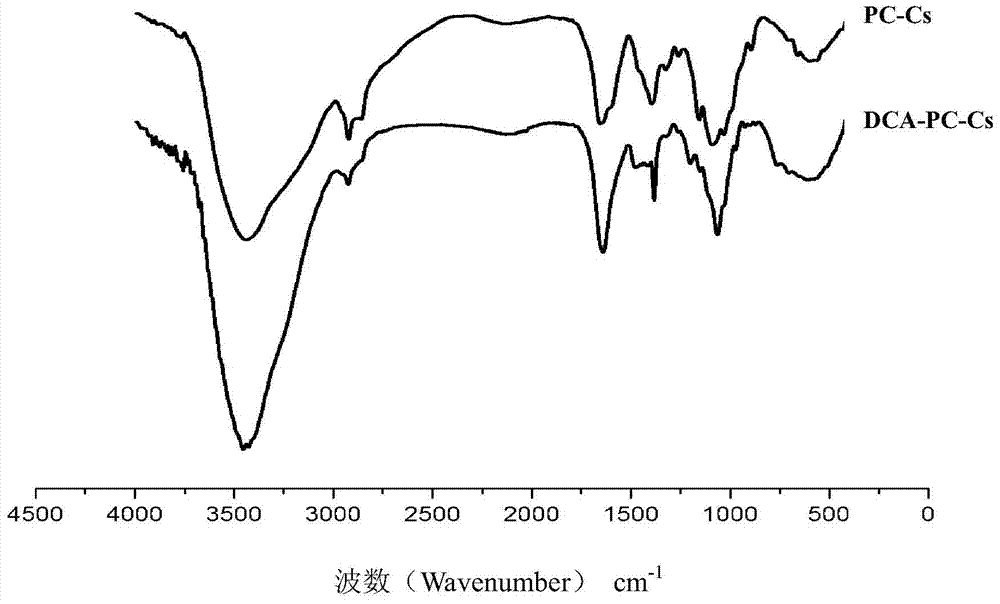
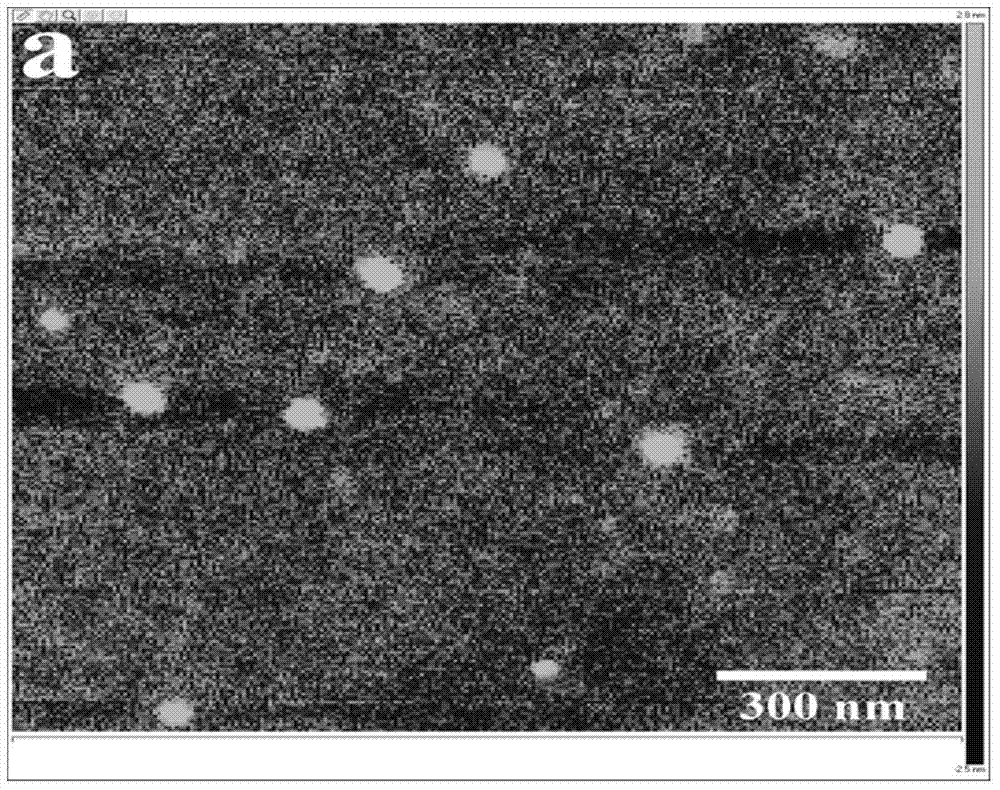
Hydrophobic modified choline phosphorylated chitosan self-assembled nano microparticle and preparation method thereof
A technology of chitosan and phosphorylcholine, which is applied in the field of hydrophobically modified phosphorylcholine chitosan self-assembled nanoparticles and its preparation, can solve the limitation of the ability to enter cells across the membrane and affect the drug delivery effect, etc. problems, achieve good cytocompatibility and blood compatibility, avoid adverse biological reactions, and inhibit non-specific protein adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of deoxycholic acid phosphorylcholined chitosan derivative nanoparticles.
[0042] Preparation steps:
[0043] 1. Take 1g of chitosan and 2.72g of phthalic anhydride in 100mL of anhydrous dimethylformamide, under the protection of nitrogen, react at 130°C for 8 hours, after filtering, the clear liquid is precipitated with ice water and dried to obtain phthalic anhydride. Phthalated chitosan. Take 1g of phthalylated chitosan and 9.58g of triphenylchloromethane in 25mL of anhydrous pyridine, under nitrogen protection, react at 90°C for 24 hours, precipitate with ethanol, wash, add 100mL of hydrazine hydrate, nitrogen protection, React at 80°C for 16 hours, spin dry excess hydrazine hydrate, wash with deionized water, ethanol, ether in turn, and dry to obtain 6-O-triphenylmethyl etherified chitosan (CsTr).
[0044] 2. Dissolve 200mg of 6-O-triphenylmethyl etherified chitosan (CsTr) in 10mL of anhydrous dimethylacetamide, and add 0.42mL of triethylam...
Embodiment 2
[0057] Example 2 Preparation of Cholesterol Hemisuccinate-Phosphocholine Chitosan Derivative Nanoparticles
[0058] 1. Take 1g of chitosan and 2.72g of phthalic anhydride in 100mL of anhydrous dimethylformamide, under the protection of nitrogen, react at 130°C for 8 hours, after filtering, the clear liquid is precipitated with ice water and dried to obtain phthalic anhydride. Phthalated chitosan. Take 1g of phthalylated chitosan and 9.58g of triphenylchloromethane in 25mL of anhydrous pyridine, under nitrogen protection, react at 90°C for 24 hours, precipitate with ethanol, wash, add 100mL of hydrazine hydrate, nitrogen protection, React at 80°C for 16 hours, spin dry excess hydrazine hydrate, wash with deionized water, ethanol, ether in turn, and dry to obtain 6-O-triphenylmethyl etherified chitosan (CsTr).
[0059] 2. Dissolve 200mg of 6-O-triphenylmethyl etherified chitosan (CsTr) in 10mL of anhydrous dimethylacetamide, and add 0.42mL of triethylamine and 0.19mL of CCl at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com