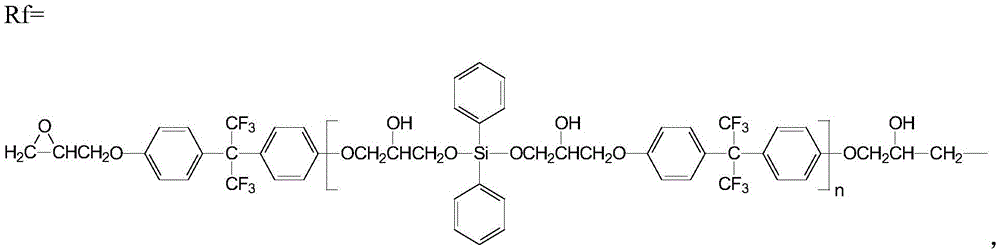
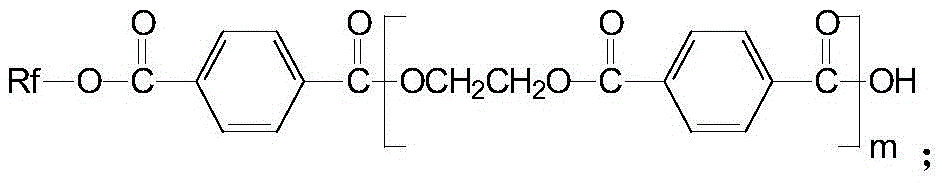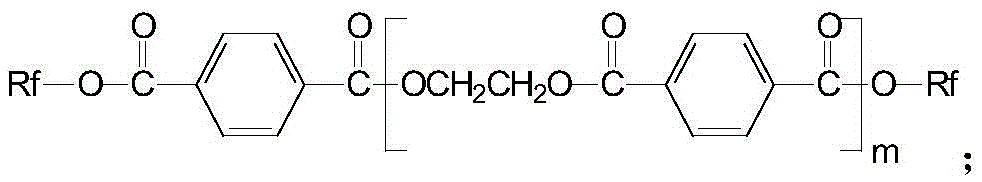A kind of anti-creep anti-hydrolysis polyester and preparation method thereof
An anti-hydrolysis and anti-creep technology, applied in the direction of single-component polyester rayon, melt spinning, rayon manufacturing, etc., to achieve the effect of improving hydrolysis resistance, hydrolysis resistance and creep resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol diglycidyl ether:
[0055] In a nitrogen atmosphere, mix epichlorohydrin and 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylidene]diphenol at a molar ratio of 4:1 , add 1.5% of 15mol / L sodium hydroxide solution of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl) ethylene] diphenol mass, at room temperature Stir the reaction for 16h; then cool to room temperature, then add 30% of the mass of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol with anhydrous carbonic acid Sodium-saturated 5mol / L sodium hydroxide solution, stirred and reacted at room temperature for 10h; then extracted with chloroform, and the resulting organic phase was evaporated to remove chloroform and excess epichlorohydrin to obtain a thick liquid, which was added to anhydrous Recrystallization in ethanol to obtain crystals of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol diglycidyl ether;
[0056] Preparation of ...
Embodiment 2
[0060] Preparation of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol diglycidyl ether:
[0061] In a nitrogen atmosphere, mix epichlorohydrin and 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylidene]diphenol at a molar ratio of 4:1 , add 2.0% 10mol / L sodium hydroxide solution of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol mass, at room temperature Stir the reaction for 18 hours; cool to room temperature, then add 40% of the mass of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol with anhydrous sodium carbonate Saturated 3mol / L sodium hydroxide solution, stirred and reacted at room temperature for 15h; then extracted with chloroform, the resulting organic phase was evaporated to remove chloroform and excess epichlorohydrin to obtain a thick liquid, which was added to absolute ethanol Medium recrystallization, namely obtain the crystal of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol diglycidyl ether;
[0062] Preparation of fl...
Embodiment 3
[0066] Preparation of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol diglycidyl ether:
[0067] In a nitrogen atmosphere, mix epichlorohydrin and 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylidene]diphenol at a molar ratio of 4:1 , add 1.8% of 12mol / L sodium hydroxide solution of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol mass, at room temperature Stir the reaction for 17h; cool to room temperature, then add 35% of the mass of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol with anhydrous sodium carbonate Saturated 4mol / L sodium hydroxide solution, stirred and reacted at room temperature for 13h; then extracted with chloroform, the resulting organic phase was evaporated to remove chloroform and excess epichlorohydrin to obtain a thick liquid, which was added to absolute ethanol Medium recrystallization, namely obtain the crystal of 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylene]diphenol diglycidyl ether;
[0068] Preparation of fluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com