A kind of benzanthracene derivative containing pyrimidine or pyrazine or triazine group and its application
A derivative, benzanthracene technology, applied in the field of new organic materials, can solve the problems of difficult film formation and molecular crystallization, etc., and achieve the effects of increased film formation, good film formation and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
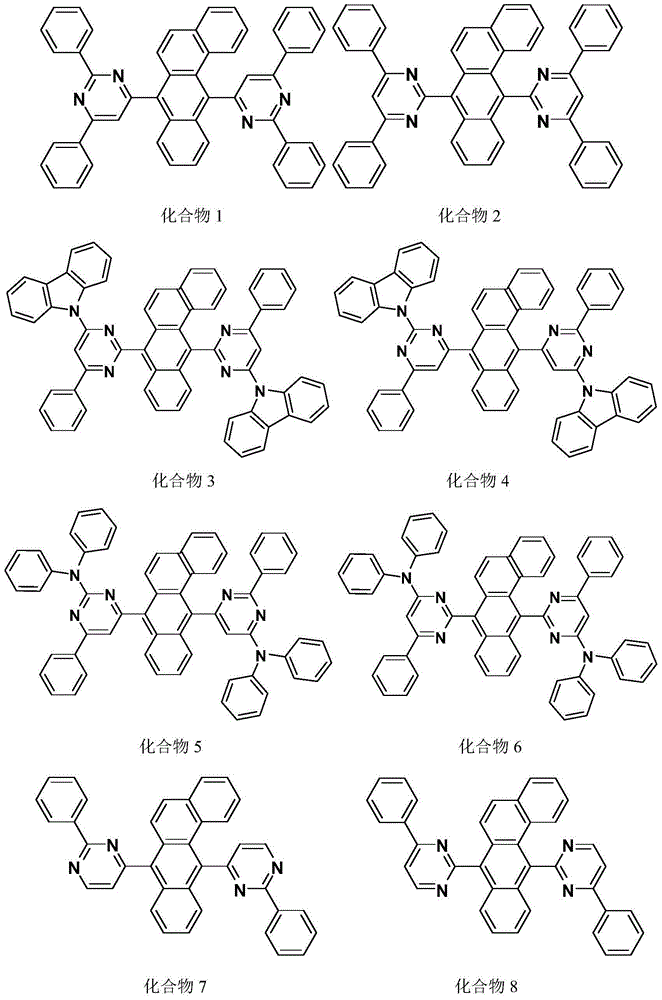
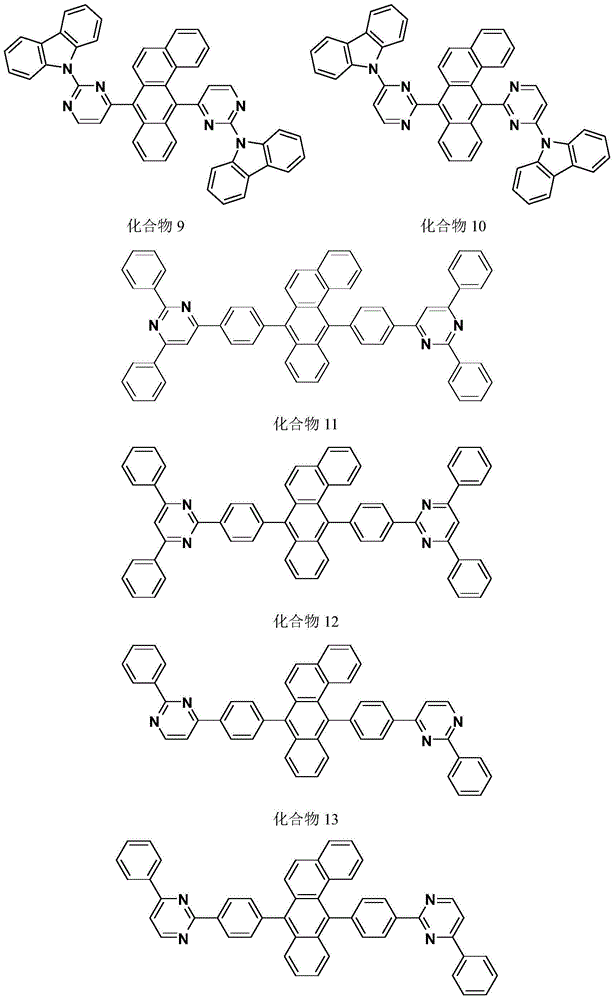
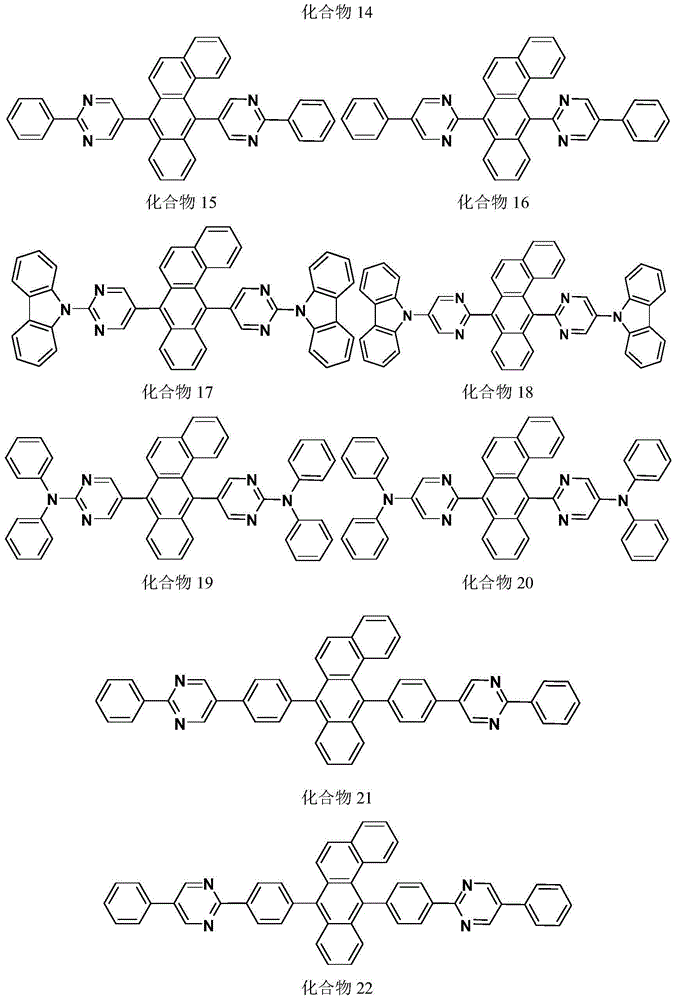
Image
Examples
Embodiment 1
[0075] The synthesis of embodiment 1 compound 1
[0076] (1) the first step
[0077]
[0078] Under the protection of Ar gas, in a 5000ml three-necked flask, 18.2g of 2,4,6-trichloropyrimidine (molecular weight 182, 0.10mol), 14.2g of benzanthracene-7,12-diboronic acid (molecular weight 316, 0.045 mol), tetrakis(triphenylphosphine) palladium 6.0g (0.0052mol), 600ml of THF, 400ml toluene, potassium carbonate 60g (0.435mol) dissolved in 400ml water to form a reaction flask. After repeated air exchange under reduced pressure, electric stirring was started, and the reaction was monitored by TLC (thin layer chromatography). After reflux for 5 hours, the reaction was complete. After cooling, the reaction system was divided into two layers. The organic layer was separated and evaporated to dryness to obtain a solid product, which was recrystallized with toluene to obtain 18.8 g of an intermediate with a molecular weight of 522 and a yield of 80%.
[0079] (2) The second step
...
Embodiment 2
[0083] The synthesis of embodiment 2 compound 2
[0084] (1) the first step
[0085]
[0086] Under the protection of Ar gas, 18.2 g of 2,4,6-trichloropyrimidine (molecular weight 182, 0.10 mol), 28.1 g of phenylboronic acid (molecular weight 122, 0.23 mol), tetrakis(triphenyl Phosphine) palladium 12.0g (0.0104mol), THF 600ml, 400ml toluene, potassium carbonate 60g (0.435mol) dissolved in 400ml water to form a solution into the reaction flask. After repeated ventilation under reduced pressure, the electric stirring was started, and the reaction was monitored by TLC (thin layer chromatography). After reflux for 8 hours, the reaction was complete. After cooling, the reaction system was divided into two layers. The organic layer was separated and evaporated to dryness to obtain a solid product, which was recrystallized with toluene to obtain 19.9 g of an intermediate with a molecular weight of 266 and a yield of 75%.
[0087] (2) The second step
[0088]
[0089] Under t...
Embodiment 3
[0091] The synthesis of embodiment 3 compound 3
[0092] (1) the first step
[0093]
[0094] Under the protection of Ar gas, carbazole 16.7g (molecular weight 167, 0.1mol) was dissolved in anhydrous DMF180ml, and a solution of 5.64g NaH (content 55%, 0.235mol) in 180ml DMF was added dropwise for 20min, stirred for 1h, and then Dissolve 18.2 g of 2,4,6-trichloropyrimidine (molecular weight 182, 0.1 mol) in 180 ml of DMF, add it in 20 min, stir for 3 h, pour into 1000 ml of water, filter the precipitate, and dry it in vacuum. The product is purified by silica gel column , 25.4 g of the target molecule (0.081 mol) was obtained with a molecular weight of 313 and a yield of 81%.
[0095] (2) The second step
[0096]
[0097] Under the protection of Ar gas, in a 5000ml three-necked flask, add 15.6g (molecular weight 313, 0.05mol) of the reaction product of the previous step, 6.71g (molecular weight 122, 0.055mol) of phenylboronic acid, tetrakis (triphenylphosphine) palladiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com