A method for producing oxygen by electrolysis of carbon dioxide
A carbon dioxide and oxygen technology, applied in the field of electrochemistry, can solve the problems of low photoelectric catalytic conversion efficiency, low reaction rate, hazards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
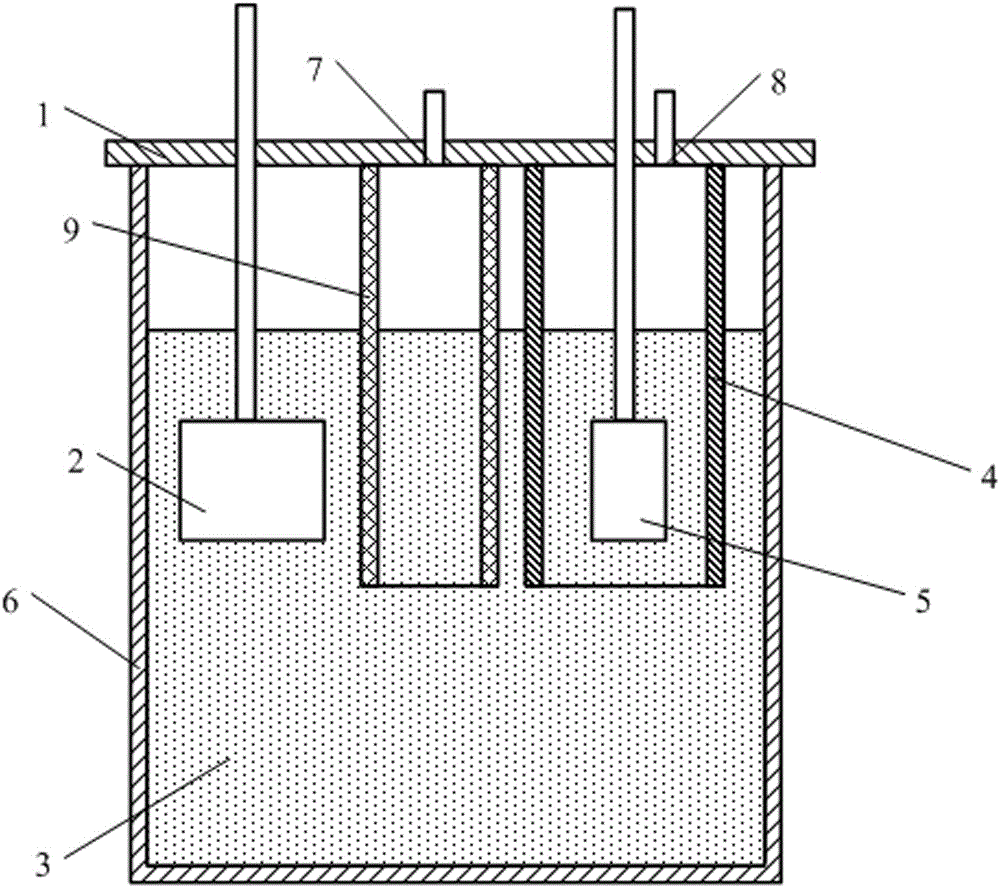
[0024] A device that electrolyzes carbon dioxide to produce oxygen, such as figure 1 As shown, the graphite crucible 6 is included, the top of the graphite crucible 6 is provided with a nickel crucible cover 1, and the nickel crucible 1 cover is provided with CO 2 Inlet 7 and oxygen outlet 8, also be provided with CO on the bottom surface of nickel crucible lid 1 2 Channel 9 and anode cover 4, CO 2 Import 7 with CO 2 Channel 9 communicates, oxygen outlet 8 communicates with anode cover 4; anode 5 and cathode 2 are inserted in graphite crucible 6 and anode 5 is inserted in anode cover 4;
[0025] Combine anhydrous LiF and Li 2 CO 3 Mix evenly at a molar ratio of 0.5:1 to obtain a mixed material;
[0026] Add additives to the mixed material and mix uniformly to make electrolyte molten salt; the additive accounts for 2% of the total weight of the electrolyte molten salt; the additive is LiCl;
[0027] Using the above device, the graphite crucible is used as the electrolytic...
Embodiment 2
[0030] The device for preparing oxygen by electrolysis of carbon dioxide is the same as in Example 1;
[0031] Combine anhydrous LiF and Li 2 CO 3 Mix evenly at a molar ratio of 1.5:1 to obtain a mixed material;
[0032] Add additives to the mixed material and mix uniformly to make electrolyte molten salt; the additive accounts for 15% of the total weight of the electrolyte molten salt; the additive is NaF;
[0033] Using the above device, the graphite crucible is used as the electrolytic cell, the electrolyte molten salt is placed in the electrolytic cell, heated to 690~700°C, and the CO 2 Cylinders and CO 2 Inlet connectivity, then through the CO 2 The channel feeds CO into the electrolyte molten salt 2 gas, and Ni electrode is used as cathode, Fe-Ni alloy electrode is used as anode, and the electrolyte molten salt is energized for electrolysis; the current density is controlled at 0.1A / cm during electrolysis 2 , the incoming CO 2 The pressure of the gas is 0.1~0.12MP...
Embodiment 3
[0036] The device for preparing oxygen by electrolysis of carbon dioxide is the same as in Example 1;
[0037] Combine anhydrous LiF and Li 2 CO 3 Mix evenly at a molar ratio of 1:1 to obtain a mixed material;
[0038] Add additives to the mixed material and mix uniformly to make electrolyte molten salt; the additive accounts for 3% of the total weight of the electrolyte molten salt; the additive is KF;
[0039] Using the above device, the graphite crucible is used as the electrolytic cell, the electrolyte molten salt is placed in the electrolytic cell, heated to 690~700°C, and the CO 2 Cylinders and CO 2 Inlet connectivity, then through the CO 2 The channel feeds CO into the electrolyte molten salt 2 Gas, and use Ni electrode as cathode, using Fe-Ni-Al 2 o 3 The alloy electrode is the anode, and the electrolyte molten salt is energized for electrolysis; the current density is controlled at 0.2A / cm during electrolysis 2 , the incoming CO 2 The pressure of the gas is 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com