Preparation method for rifampin by using micro-reaction apparatus
A technology of micro-reaction device and micro-reactor, which is applied in the direction of organic chemistry, can solve the problems of unsatisfactory product quality of rifampicin, and achieve the effect of high-efficiency heat and mass transfer capacity, good product quality and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
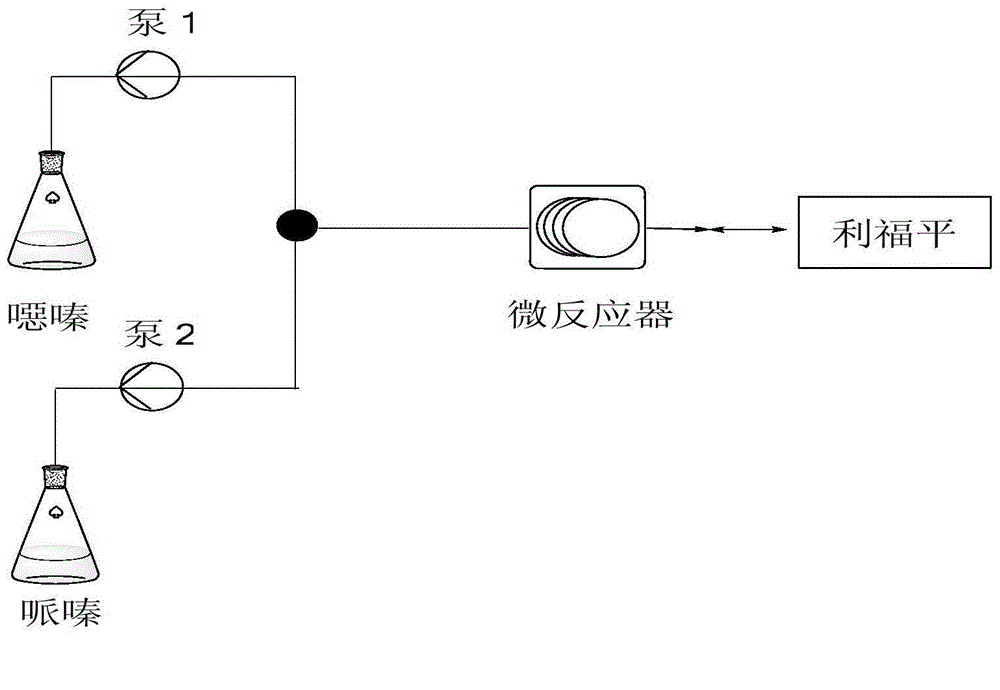
[0025] In the microchannel reaction device, the diameter of the connecting pipe is 2.1mm, the length of the inlet pipe is 15cm, the length of the connecting pipe between the T-shaped valve and the microchannel reactor is 25cm, and the length of the connecting pipe between the microreactor and the outlet is 20cm; the volume of the microchannel reactor is 5ml.
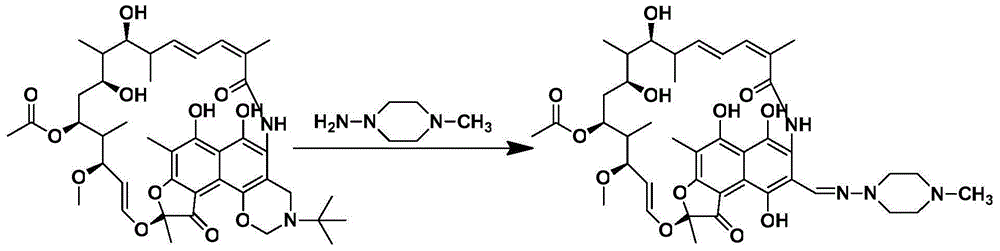
[0026] 1. Take 10.75g of rifamycin oxazine, stir it into a homogeneous phase with DMF at room temperature, and obtain a mixed solution volume of 25mL; 2. Take 0.80g of 1-methyl-4-nitropiperazine with a pipette gun, add DMF to dilute to 25mL. The two materials are mixed and pumped into the microchannel reactor through the T-valve. The two reaction fluids flow through the microreactor at 0.5mL / min, the reaction molar ratio of rifamycin oxazine and 1-methyl-4-nitropiperazine is 1:1, and the reactor temperature is 40°C. The reaction residence time was 5 min, and the crude product was collected at the outlet; the conversion...
Embodiment 2
[0028] The microchannel reactor is basically the same as Example 1, and the difference is only: the pipeline diameter of this microchannel reactor is 2.1mm, and the length of the liquid inlet pipe is 10cm, and the connecting pipe length between the inlet and the microreactor The length of the connecting tube between the microreactor and the outlet is 30cm; the volume of the microchannel reactor is 4ml.
[0029] 1. Take 10.75g of rifamycin oxazine, stir it with tetrahydrofuran at room temperature to form a homogeneous phase, and the volume of the mixed solution is 30mL; 2. Take 0.80g of 1-methyl-4-nitropiperazine with a pipette gun, add tetrahydrofuran to dilute to 30mL. The materials are mixed and pumped into the microchannel reactor through the T-valve. The two reaction fluids flowed through the microreactor at 0.4mL / min, the reaction molar ratio of rifamycin oxazine and 1-methyl-4-nitropiperazine was 1:1, and the reactor temperature was 50°C. The reaction residence time wa...
Embodiment 3
[0031] The microchannel reactor is basically the same as Example 1, and the difference is only: the pipeline diameter of this microchannel reactor is 1.6mm, and the liquid inlet pipe length is 8cm, and the connecting pipe length between the inlet and the microreactor The length of the connecting tube between the microreactor and the outlet is 25cm; the volume of the microchannel reactor is 6mL.
[0032] 1. Take 10.75g of rifamycin oxazine, stir it with acetic acid at room temperature to form a homogeneous phase, and the volume of the mixed solution is 20mL; 2. Take 0.80g of 1-methyl-4-nitropiperazine with a pipette gun, add acetic acid to dilute to 20mL. The materials are mixed and pumped into the microchannel reactor through the T-valve. The two reaction fluids flowed through the microreactor at 0.4mL / min, the reaction molar ratio of rifamycin oxazine and 1-methyl-4-nitropiperazine was 1:1, and the reactor temperature was 60°C. The reaction residence time was 6.25 min. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com