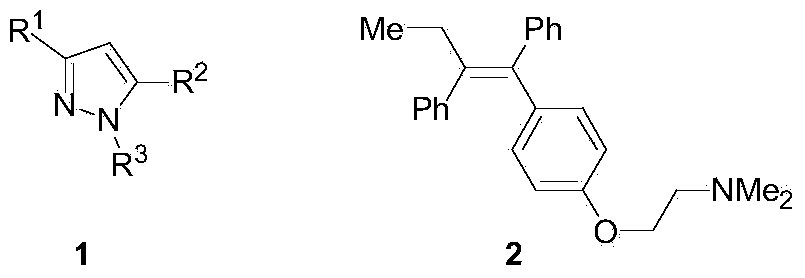
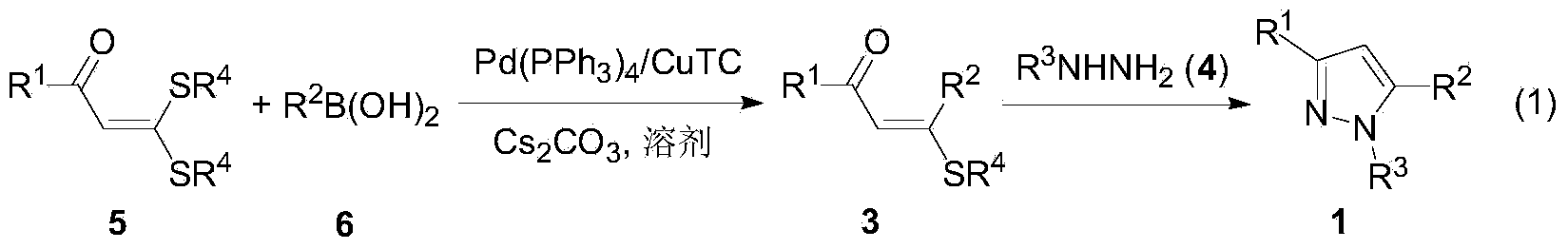
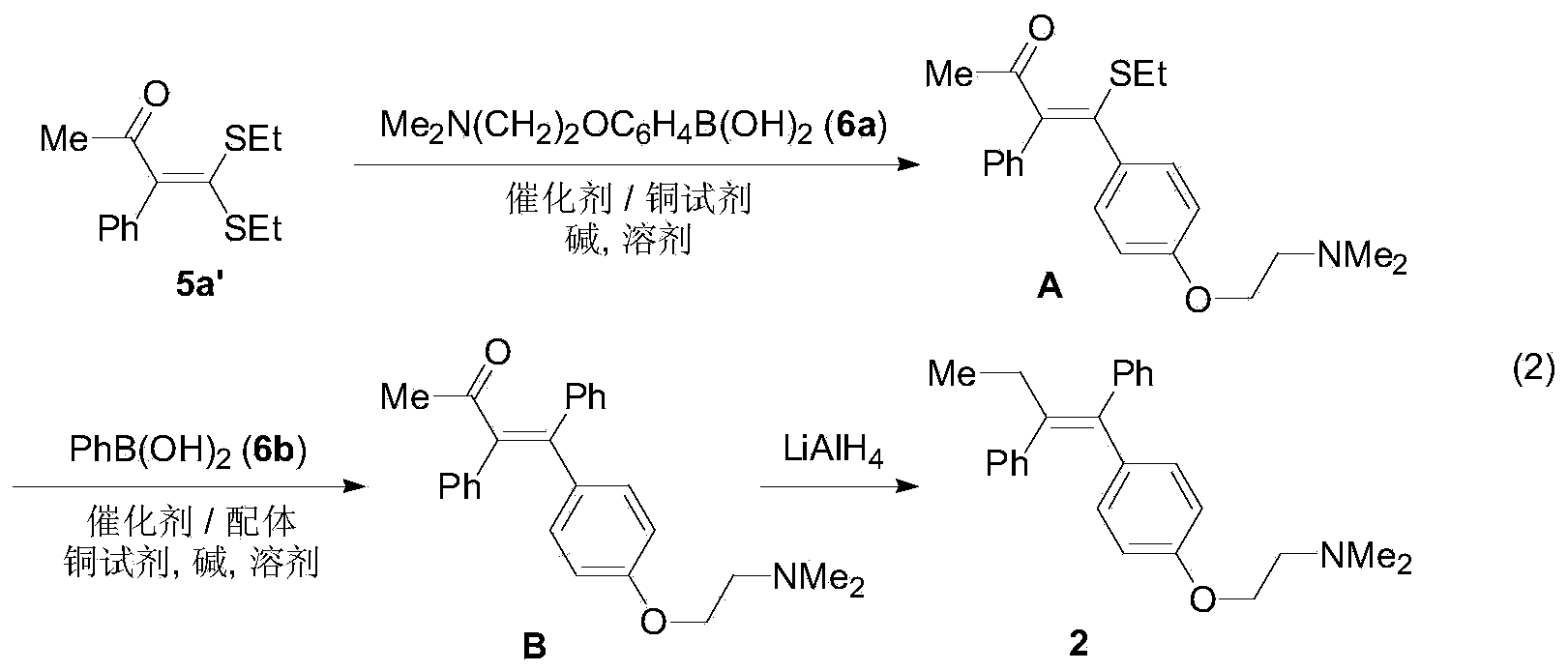
Preparation method of tetra-substituted olefin and its pyrazole derivatives
A tetra-substituted, alkene technology, used in the preparation of organic compounds, the preparation of aminohydroxy compounds, chemical instruments and methods, etc., can solve the problems of poor stereoselectivity, harsh reaction conditions, and complicated preparation routes, and achieve simple steps and reaction conditions. Mild, inexpensive and easily available effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Under nitrogen atmosphere, α-carbonyl dithioketene 5b (95mg, 0.50mmol), arylboronic acid 6b (91mg, 0.75mmol), Pd(PPh 3 ) 4 (43mg, 0.0375mmol), CuTC (191mg, 1.0mmol), Cs 2 CO 3 (326mg, 1.0mmol) and 5mL solvent THF, stirred at 50°C for 2h. After the reaction was finished, the mixture was cooled to room temperature, the reaction solution was filtered with diatomaceous earth, the filter cake was washed with 10 mL of dichloromethane, the filtrate was decompressed to remove volatile components, and then separated by silica gel column chromatography (eluent was petroleum ether ( 60-90°C) / ethyl acetate, v / v=30:1), the yellow liquid intermediate 3a was obtained (100 mg, yield 97%). Intermediate products were confirmed by NMR and high-resolution mass spectrometry.
[0032] Under a nitrogen atmosphere, add 3a (103mg, 0.50mmol), phenylhydrazine 4a (65mg, 0.60mmol), potassium tert-butoxide (112mg, 1.0mmol) and 5mL solvent tert-butanol to a 25mL reaction flask in sequ...
Embodiment 2
[0034] The reaction steps and operations are the same as those in Example 1, except that the reaction temperature in the second step is room temperature 25° C., and the reaction time is 24 hours. The reaction was stopped, and the target product 1a (10 mg, yield 9%) was obtained through the same post-treatment as above. It shows that the reaction temperature decreases and the reaction becomes slower.
Embodiment 3
[0036] The reaction steps and operations are the same as in Example 1, except that the second step reaction solvent is toluene. The reaction was stopped, and the target product 1a (30 mg, yield 26%) was obtained after post-processing. It shows that the use of aprotic solvent is not conducive to the condensation and cyclization reaction of 3 and hydrazine 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com