Alpha-hydroxy unsaturated alkylphosphonic acid compound as well as preparation method and application of alpha-hydroxy unsaturated alkylphosphonic acid compound
An alkyl phosphonic acid, unsaturated technology, applied in the field of mineral flotation collector compound and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
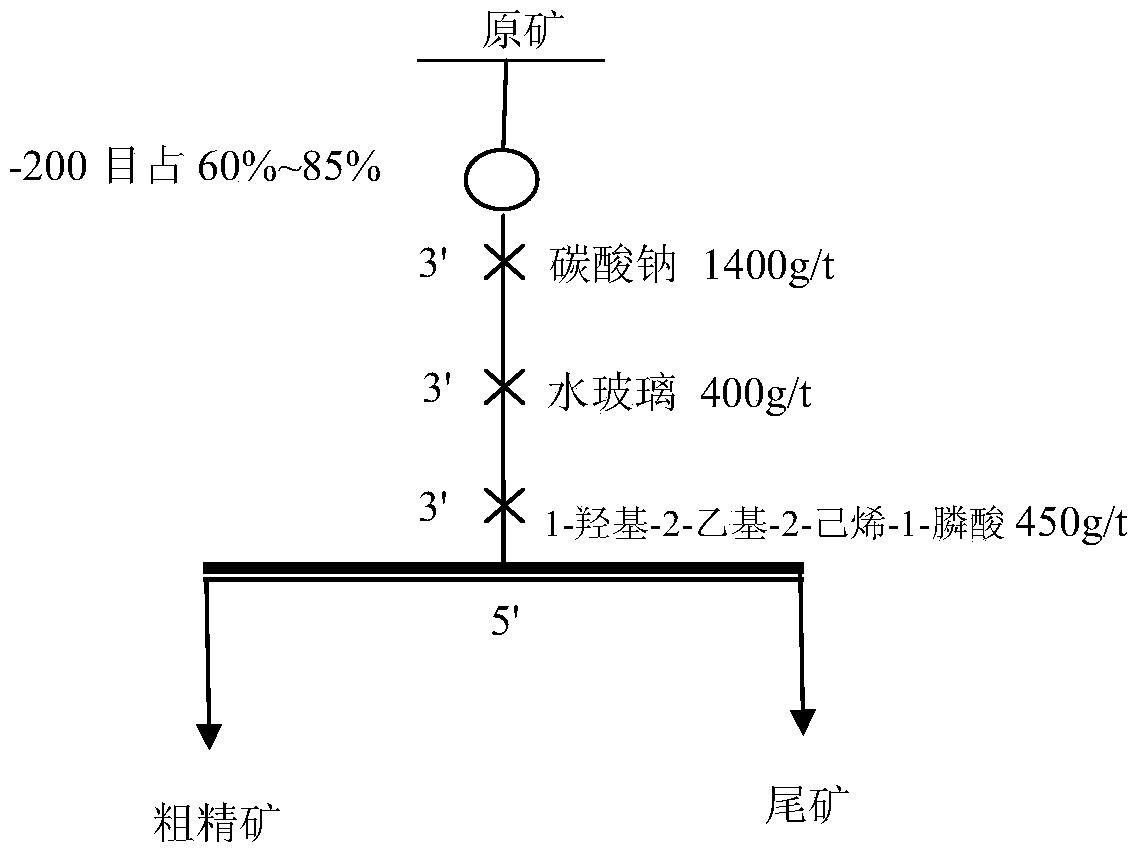
Examples
Embodiment 1
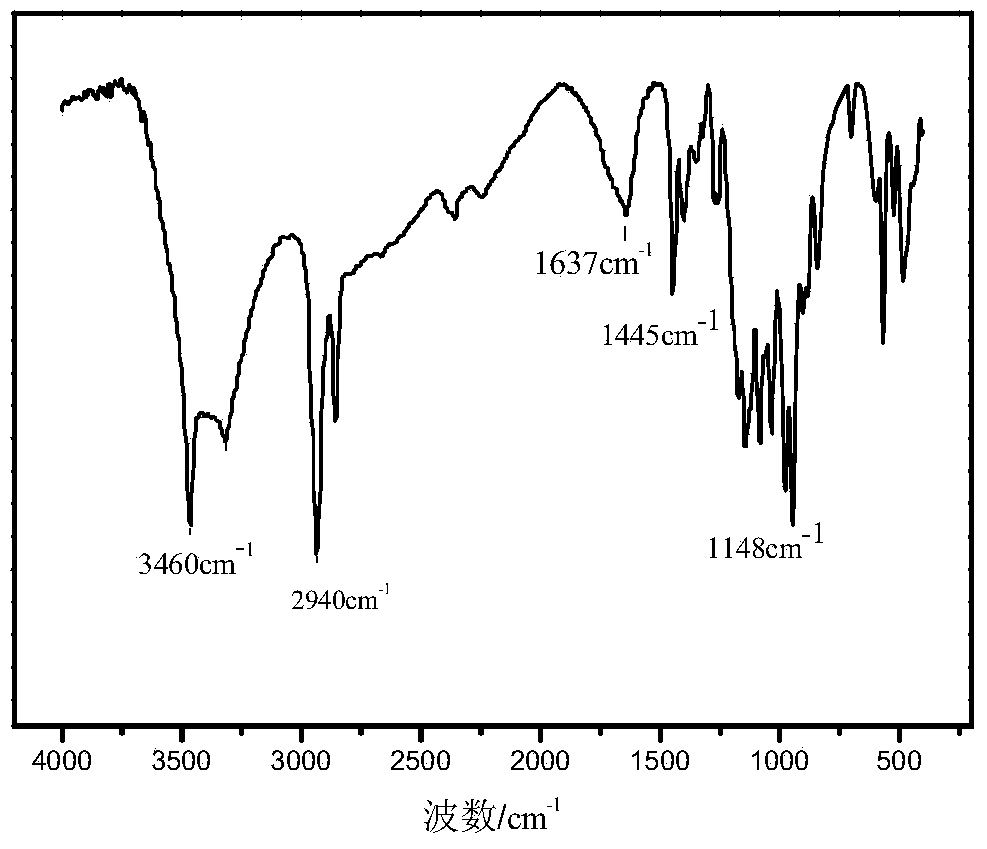
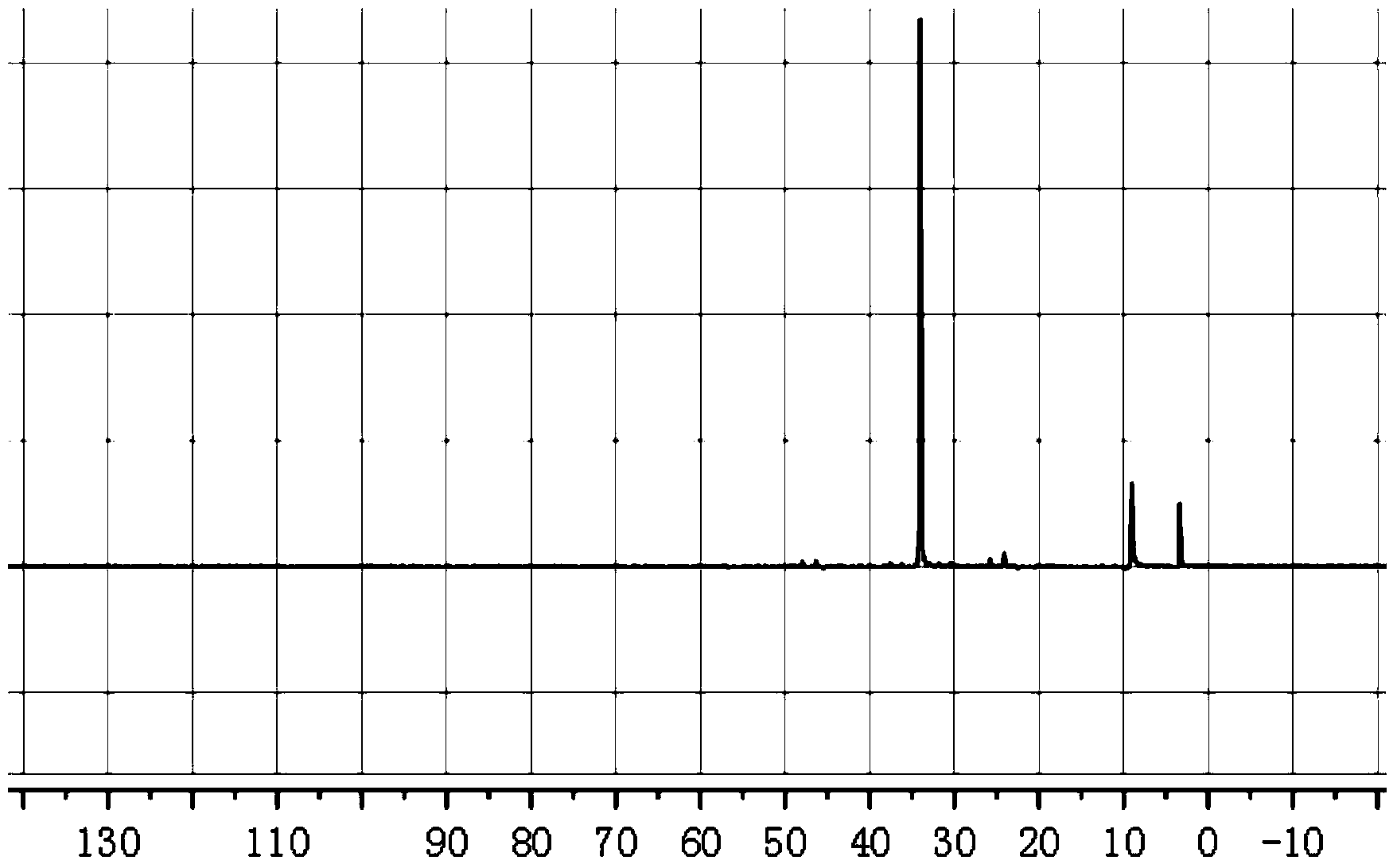
[0031] Example 1 Preparation of 1-hydroxyl-2-ethyl-2-hexene-1-phosphonous acid
[0032] Fully mix 4.4 parts of 2-ethyl-2-hexenal, 40 parts of 1,4-dioxane, 7 parts of hypophosphorous acid, and 2 parts of 20% dilute sulfuric acid, stir at 80°C for 5 hours, and use 1.4 parts of ethyl acetate Ester extraction of 1-hydroxy-2-ethyl-2-hexene-1-phosphonous acid, the extraction temperature is 30 ° C, extraction 3 times, liquid separation, take the upper liquid and distill under reduced pressure to remove ethyl acetate, the distillation temperature is 50 °C, the vacuum degree is 0.098MPa, the residue is 1-hydroxy-2-ethyl-2-hexene-1-phosphonous acid, and the yield is 75.2%.
Embodiment 2
[0033] Example 2 Preparation of 1-hydroxyl-2-ethyl-2-hexene-1-phosphonous acid
[0034] Fully mix 4.4 parts of 2-ethyl-2-hexenal, 40 parts of 1,4-dioxane, 7 parts of hypophosphorous acid, and 1 part of 37% concentrated hydrochloric acid, stir and react at 80°C for 5 hours, and use 1.4 parts of ethyl acetate Ester extraction of 1-hydroxy-2-ethyl-2-hexene-1-phosphonous acid, the extraction temperature is 30 ° C, extraction 3 times, liquid separation, take the upper liquid and distill under reduced pressure to remove ethyl acetate, the distillation temperature is 50 °C, the vacuum degree is 0.098MPa, the residue is 1-hydroxy-2-ethyl-2-hexene-1-phosphonous acid, and the yield is 83.5%.
Embodiment 3
[0035] Example 3 Preparation of 1-hydroxyl-2-ethyl-2-hexene-1-phosphonous acid
[0036]Mix 4.4 parts of 2-ethyl-2-hexenal, 40 parts of ethanol, 7 parts of hypophosphorous acid, and 1 part of hydrochloric acid, stir and react at 80°C for 5 hours, and extract 1-hydroxy-2-ethyl with 1.4 parts of ethyl acetate -2-hexene-1-phosphonous acid, the extraction temperature is 30°C, extract 3 times, separate the liquid, take the upper liquid and distill under reduced pressure to remove ethyl acetate, the distillation temperature is 55°C, the vacuum degree is 0.098MPa, the residue That is 1-hydroxy-2-ethyl-2-hexene-1-phosphonous acid with a yield of 70.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com