Cefixime dry suspension and preparation method thereof
A cefixime, dry suspension technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as the difficulty of clinical selection, and achieve the guarantee of efficacy and safety, high economic and social Meaning, the effect of accelerating absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
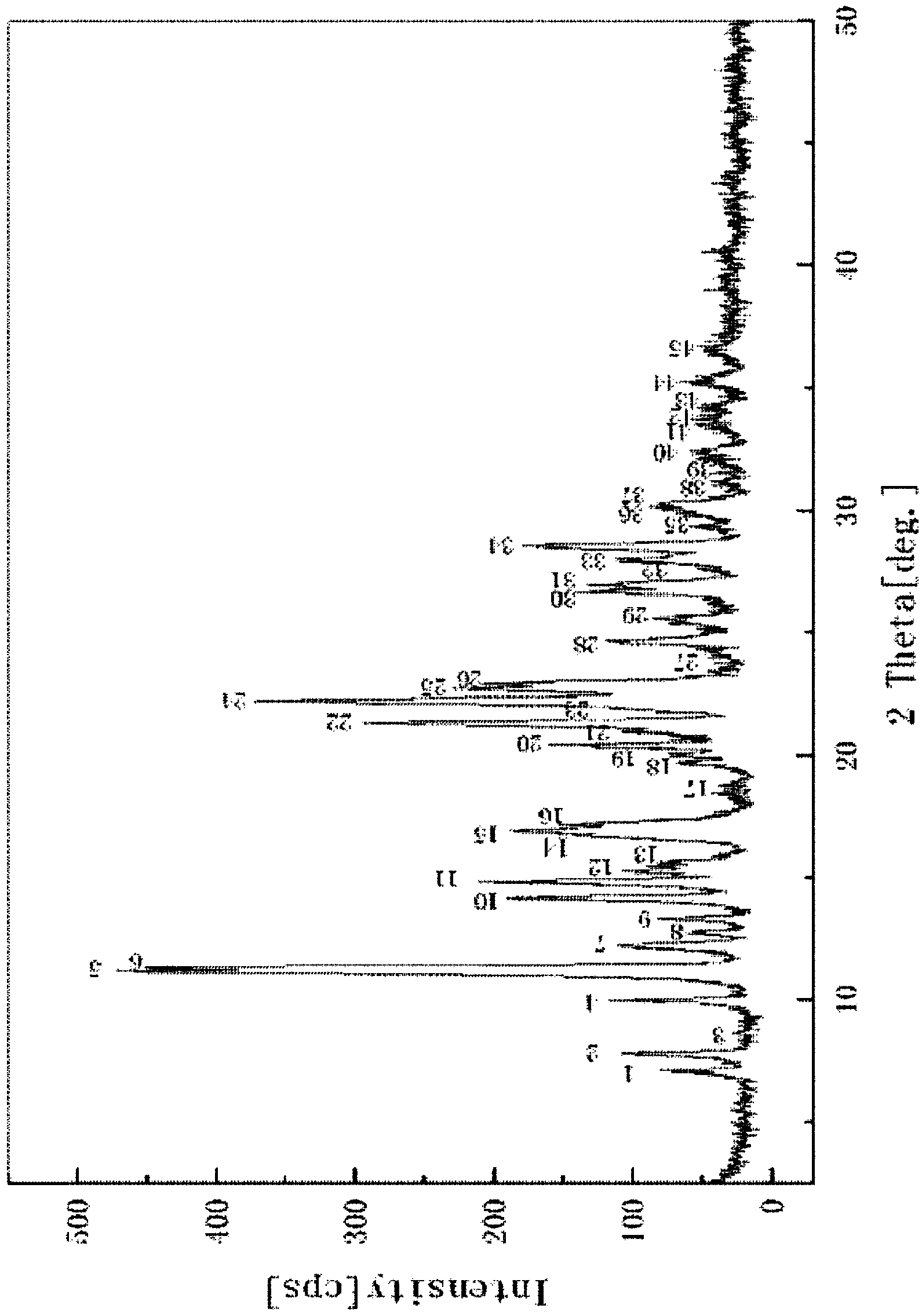
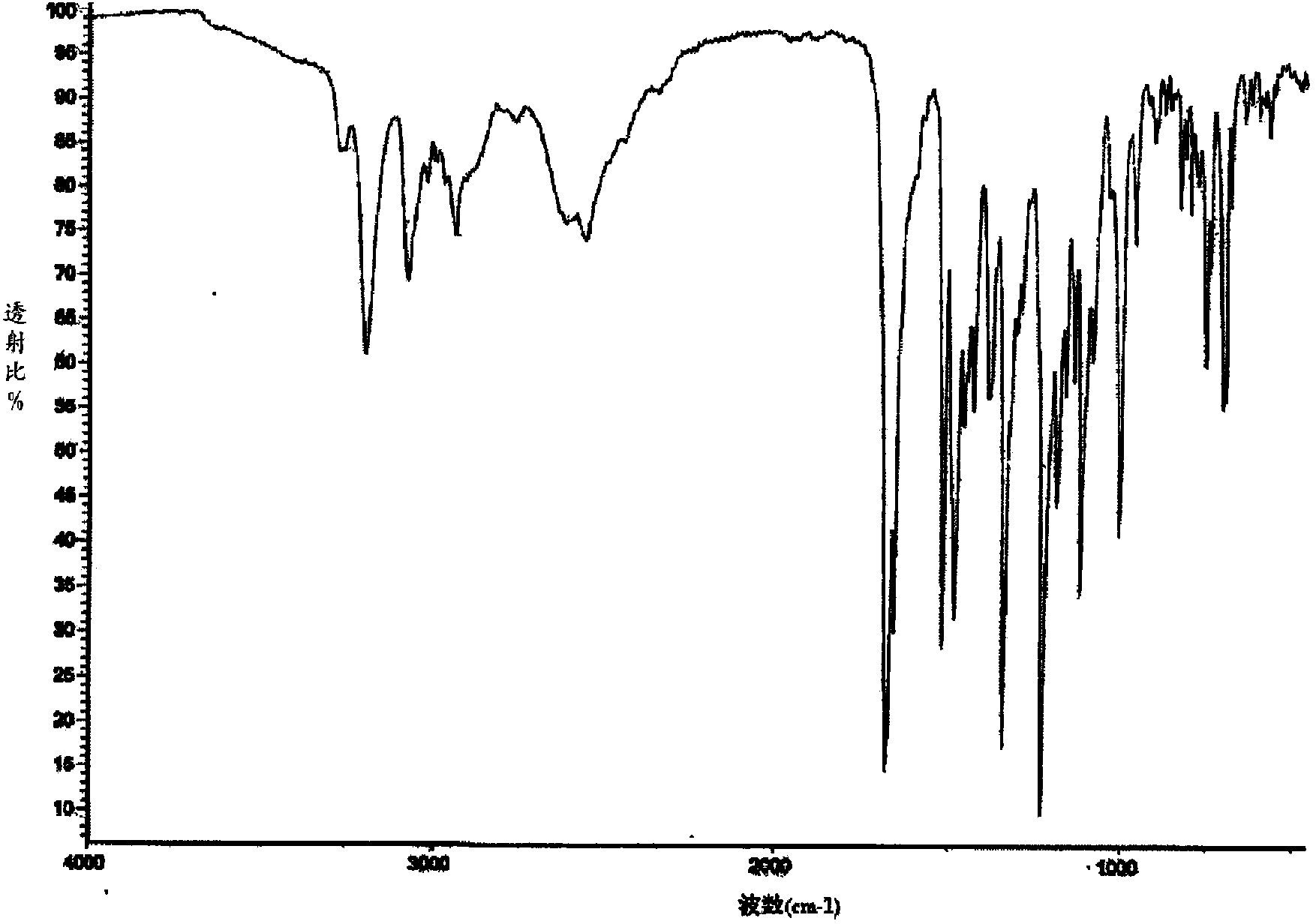
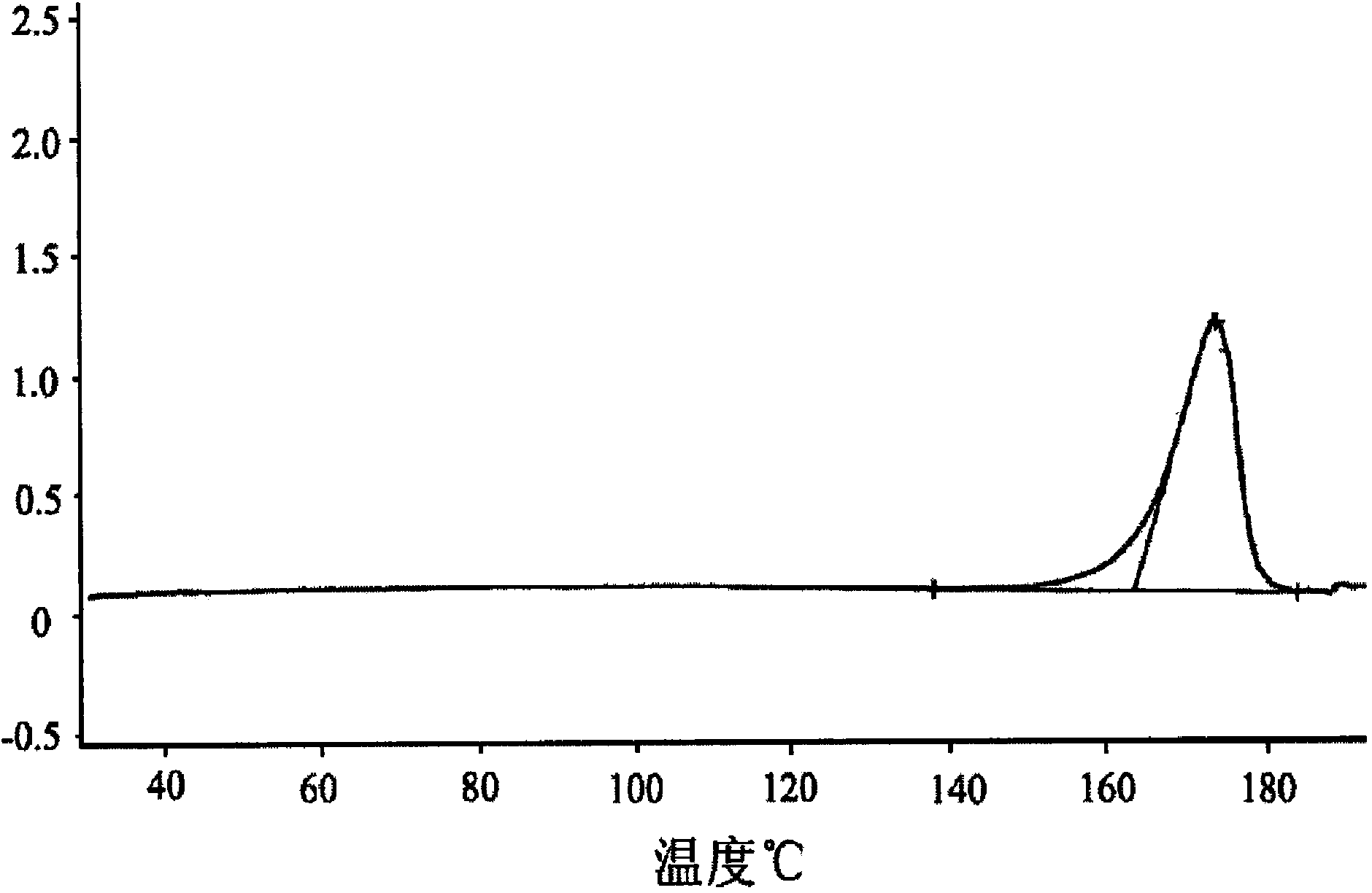
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of Cefixime Dry Suspension
[0024] prescription:
[0025]
[0026] Preparation:
[0027] a. Preparation of cefixime crystal form: dissolve 100g cefixime in 800ml dioxane and warm to 53°C, adjust the pH value to 2.5 with 20% hydrochloric acid solution, then add 16ml n-butane within 5 minutes , keep the solution at 53°C for 20 minutes, add 2400ml of tetrahydrofuran, cool to -7°C for crystallization, filter, and dry at 53°C to obtain 96.4g of cefixime crystal form, with a yield of 96.4% and a purity of 99.57% as determined by HPLC;
[0028] b. Raw material preparation: the cefixime crystal form raw material is passed through a 60-mesh sieve, the auxiliary materials are passed through a 80-mesh sieve, and weighed according to the prescription amount;
[0029] c. Mixing: Add all the materials obtained in step b into a mixer and mix thoroughly. After checking that the semi-finished product is qualified, pack it into aluminum-plastic composite film ...
Embodiment 2
[0034] Example 2 Preparation of Cefixime Dry Suspension
[0035] prescription:
[0036]
[0037]
[0038] Preparation:
[0039] a. Preparation of cefixime crystal form: dissolve 100g cefixime in 500ml dioxane and warm to 50°C, adjust the pH value to 2.0 with 20% hydrochloric acid solution, then add 5ml n-butane within 5 minutes , keep the solution at 50°C for 20 minutes, add 1000ml of tetrahydrofuran and cool to -5°C for crystallization, filter, and dry at 50°C to obtain 95.8g of cefixime crystal form, with a yield of 95.8% and a purity of 99.38% as determined by HPLC;
[0040] b. Raw material preparation: the cefixime crystal form raw material is passed through a 60-mesh sieve, the auxiliary materials are passed through a 80-mesh sieve, and weighed according to the prescription amount;
[0041] c. Mixing: Add all the materials obtained in step b into a mixer and mix thoroughly. After checking that the semi-finished product is qualified, pack it into aluminum-plastic c...
Embodiment 3
[0042] Example 3 Preparation of Cefixime Dry Suspension
[0043] prescription:
[0044]
[0045] Preparation:
[0046] a. Preparation of cefixime crystal form: dissolve 100g cefixime in 1000ml dioxane and warm to 55°C, adjust the pH value to 3.0 with 20% hydrochloric acid solution, then add 30ml n-butane within 5 minutes , keep the solution at 55°C for 20 minutes, add 4000ml of tetrahydrofuran and cool to -10°C for crystallization, filter, and dry at 55°C to obtain 95.4g of cefixime crystal form, with a yield of 95.4% and a purity of 99.33% as determined by HPLC;
[0047] b. Raw material preparation: the cefixime crystal form raw material is passed through a 60-mesh sieve, the auxiliary materials are passed through a 80-mesh sieve, and weighed according to the prescription amount;
[0048] c. Mixing: Add all the materials obtained in step b into a mixer and mix thoroughly. After checking that the semi-finished product is qualified, pack it into aluminum-plastic composite ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com