Full-automatic rapid hepatitis B virus nucleic acid testing reagent tube and application method thereof
A hepatitis B virus and detection reagent technology, which is applied in the field of fully automatic rapid nucleic acid detection reagent tubes, can solve the problems of increasing the cost of PCR detection, showing limitations, and cumbersome procedures, etc., to eliminate the risk of false positive contamination and to operate easily OK, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
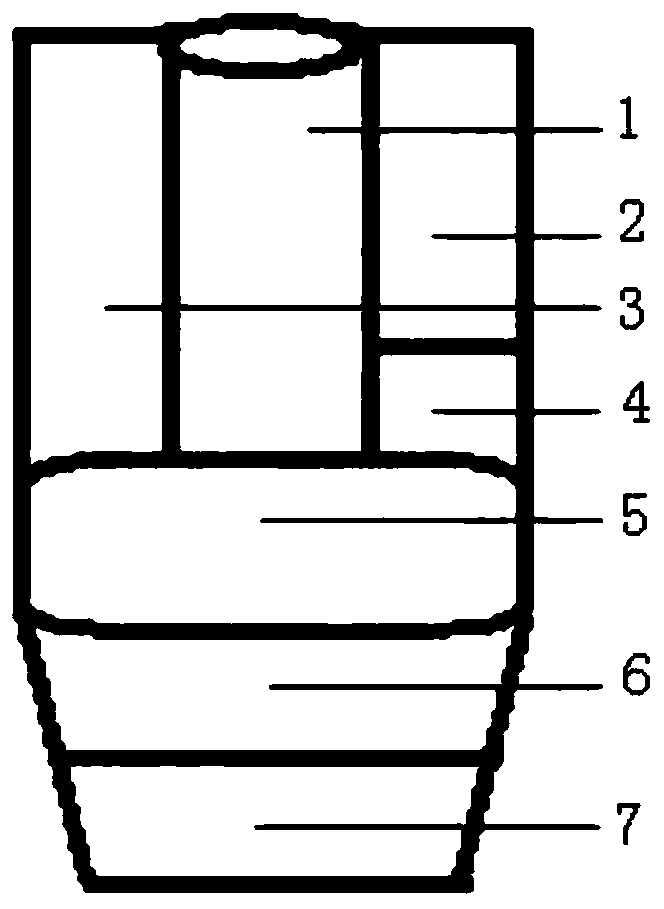
[0052] Such as figure 1 , 2 As shown, it is a fully automatic rapid nucleic acid detection reagent tube for hepatitis B virus of the present invention, including a serum sample cavity 1 for loading a serum sample to be tested and a detection reagent cavity, and the detection reagent cavity includes:
[0053] Lysis buffer chamber 2 for filling the lysis buffer,
[0054] Nucleic acid extraction magnetic bead cavity 4 for loading nucleic acid extraction magnetic beads,
[0055] Nucleic acid extraction washing liquid cavity 3 for loading nucleic acid extraction washing liquid,
[0056] Nucleic acid extraction eluent cavity 5 for loading nucleic acid extraction eluent,
[0057] Fluorescent quantitative PCR reaction solution cavity 6 for loading fluorescent quantitative PCR reaction solution,
[0058] And PCR reaction primers and probe chamber 7 for loading PCR reaction primers and probes;
[0059] The sequence of the PCR reaction primer is:
[0060] HBV1:5'-CGCACCTCTCTTTACG-3...
Embodiment 2
[0081] The fully automatic rapid nucleic acid detection reagent tube for hepatitis B virus in Example 1 of the present invention was used to detect the plasmid standard containing hepatitis B virus DNA.
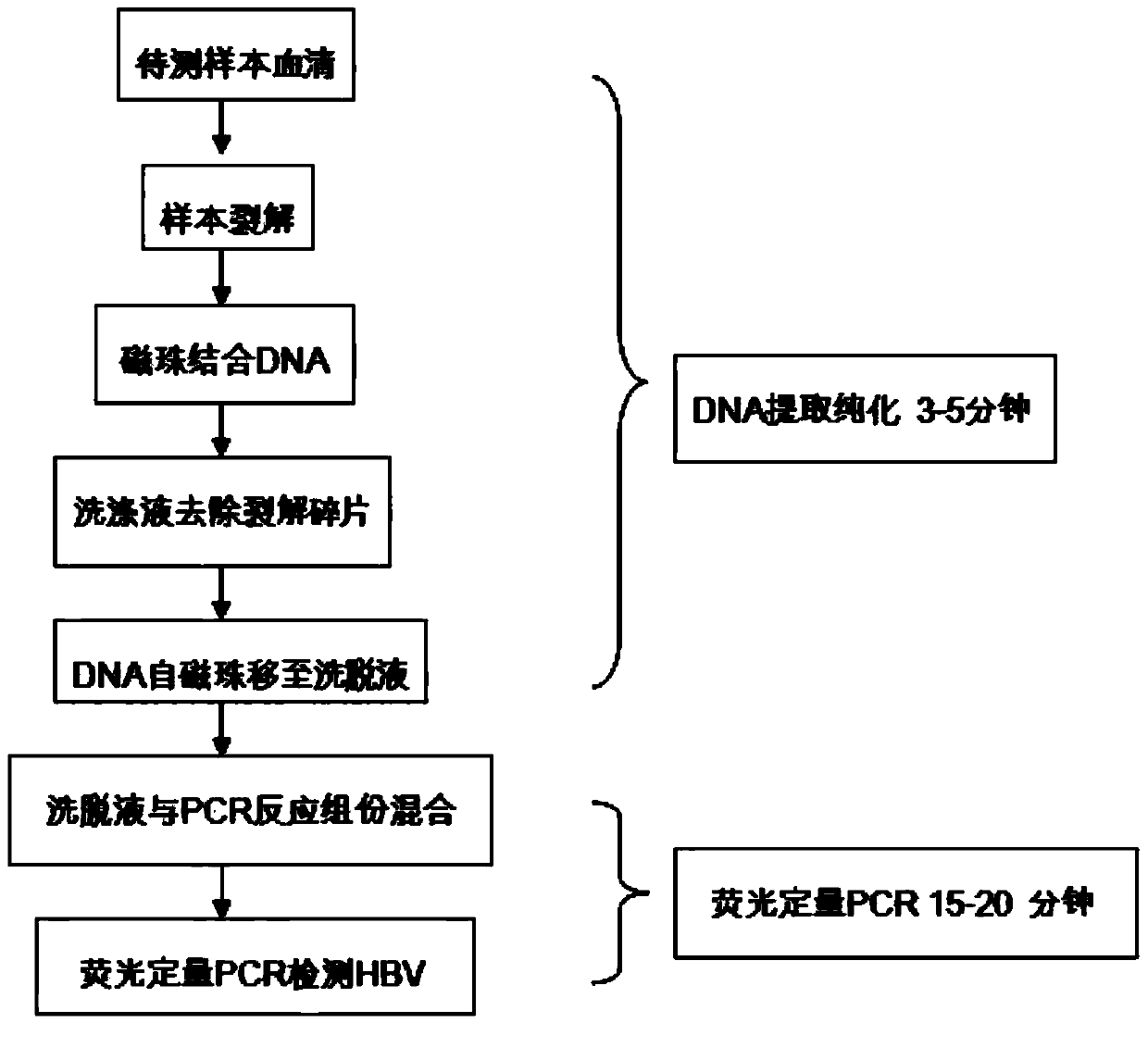
[0082] Add 50 μL of Escherichia coli liquid containing hepatitis B virus DNA plasmid standard to the fully automatic rapid nucleic acid detection reagent tube of hepatitis B virus in Example 1 of the present invention, and then put the detection reagent tube into a tube type fully automatic nucleic acid Extract the amplification instrument, set the reaction program according to the method used in Example 1, lyse for 30 seconds; magnetic beads bind for 30 seconds; wash for 40 seconds; Carry out 40 variable temperature cycles, a single variable temperature cycle includes 95° C. for 4 seconds and 60° C. for 8 seconds, and the entire reaction time is about 20 minutes to complete.
[0083] The detection data of Example 2 were compared with the following comparative experiments 1 a...
Embodiment 3
[0089] The fully automatic rapid nucleic acid detection reagent tube for hepatitis B virus in Example 1 of the present invention was used for the detection of DNA in serum samples of clinical hepatitis B patients.
[0090] 100 μL containing HBV 2 x 10 5 The copies / mL clinical serum sample is added to the fully automatic rapid nucleic acid detection reagent tube of hepatitis B virus in the embodiment 1 of the present invention, and then the detection reagent tube is put into a tube type automatic nucleic acid extraction and amplification instrument, according to the embodiment 1 How to use Set the reaction program, cleavage for 30 seconds; magnetic beads binding for 30 seconds; wash for 40 seconds; 95°C for 4 seconds and 60°C for 8 seconds, the total reaction time is about 20 minutes to complete.
[0091] The detection data of Example 3 was compared with the following comparative experiments 3 and 4. Each experiment was repeated three times, and the Ct value detection data of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com