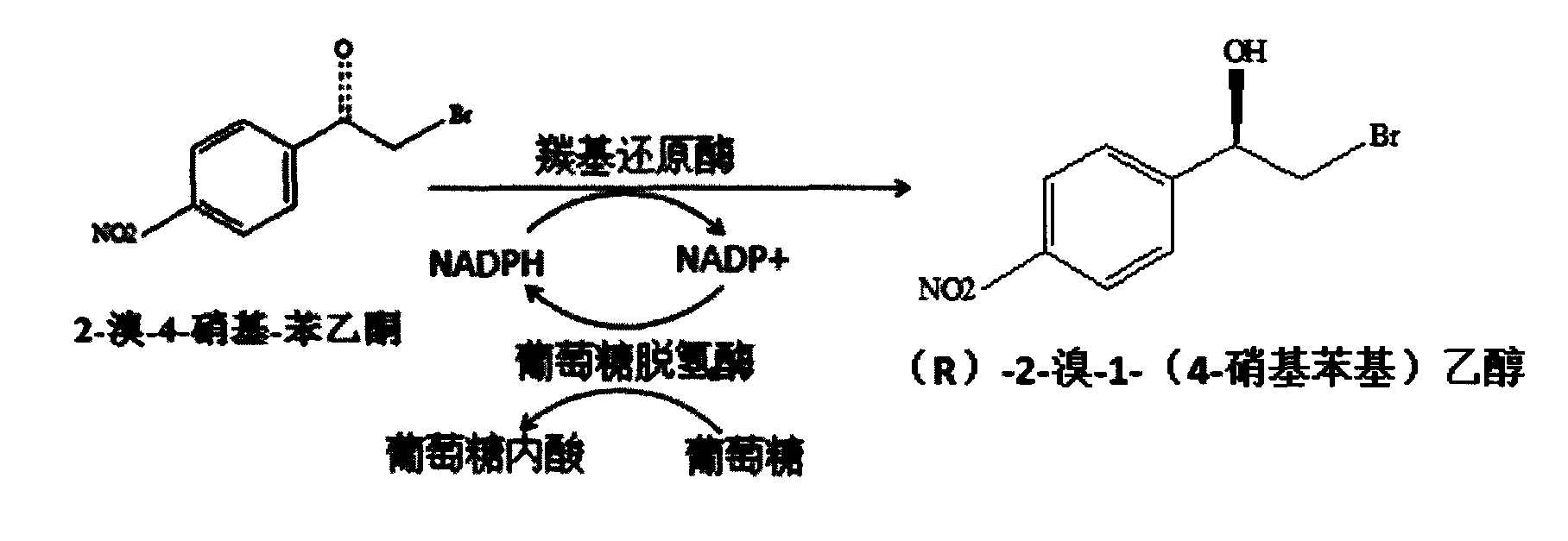
Method for preparing (R)-2-bromo-1-(4-nitrophenyl) ethanol
A technology of nitrophenyl and nitroacetophenone, which is applied in the field of bioengineering, can solve the problems of low conversion rate of substrate, low optical purity of product, recycling of coenzyme, etc., and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
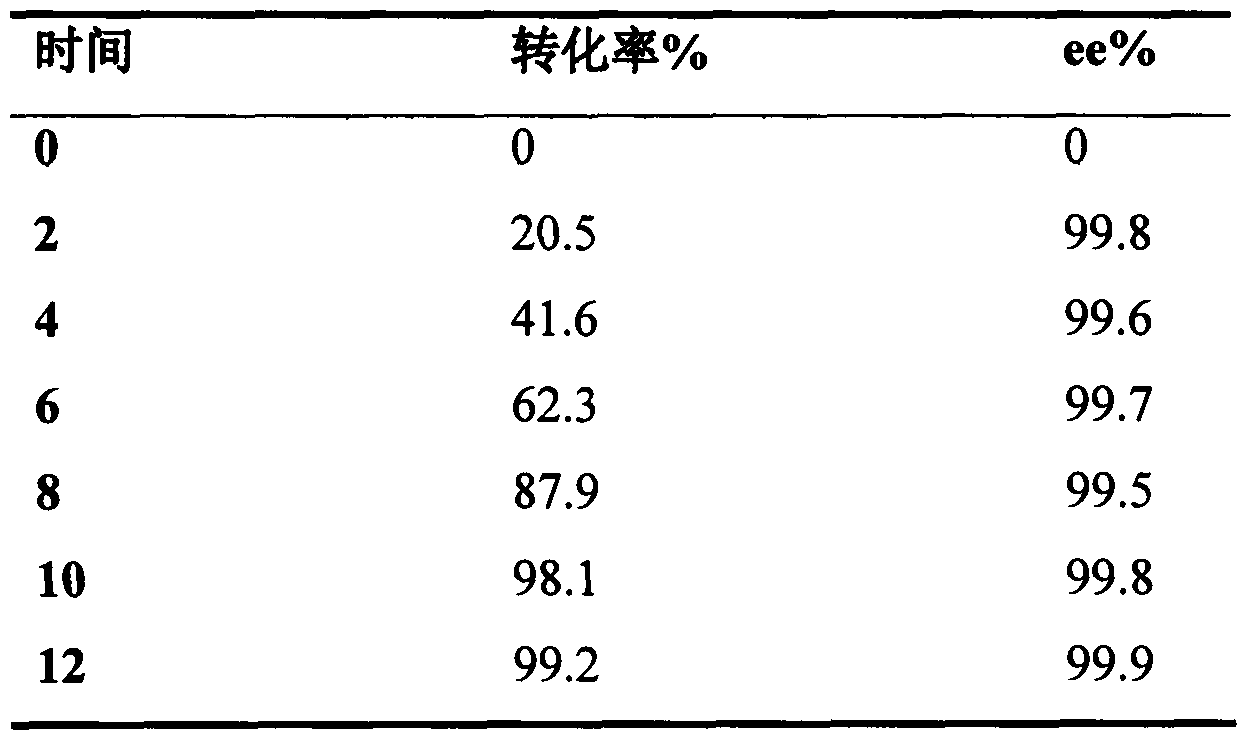
[0017] The determination of embodiment 1 enzyme activity
[0018] Carbonyl reductase (CBR) activity measurement: the reaction system is 250μl 0.1mol / L potassium phosphate buffer (pH7.0), 0.2mM NAD(P)H, 5mM substrate and appropriate amount of crude enzyme solution. Immediately after adding the enzyme solution, start scanning the change of the absorbance of the reaction system at 340nm. Enzyme activity definition: the amount of enzyme required to oxidize 1 μmol NAD(P)H per minute is one enzyme activity unit (U).
[0019] Determination of glucose dehydrogenase (GDH) activity: the reaction is 250μl 0.1mol / L potassium phosphate buffer (pH7.0), 0.2mM NADP + , 0.1M substrate glucose and appropriate amount of crude enzyme solution. Immediately after adding the enzyme solution, start scanning the change of the absorbance of the reaction system at 340nm. Enzyme activity definition: reduce 1 μmol NADP per minute + The required amount of enzyme is one enzyme activity unit (U). At the...
Embodiment 2
[0020] Embodiment 2 recombinant bacteria BL21 (pET28a-cbr), the fermentation of BL21 (pET28a-gdh)
[0021] Inoculate the recombinant bacteria BL21(pET28a-cbr) and BL21(pET28a-gdh), in 2ml of LB liquid medium containing 100μg / ml kanamycin, shake culture at 37°C, 220r / min for 12h. Transfer 300ul of the culture solution to 30ml of LB liquid medium containing 100μg / ml kanamycin and culture at 37°C with shaking at 220r / min until OD 600 When the concentration is 0.6-0.8, add IPTG to the culture to a final concentration of 1.0mmol / L, shake at 25°C and 220r / min for 10h, and collect the bacteria by centrifugation at 4°C for later use.
Embodiment 3
[0022] The detection method of embodiment 3 products
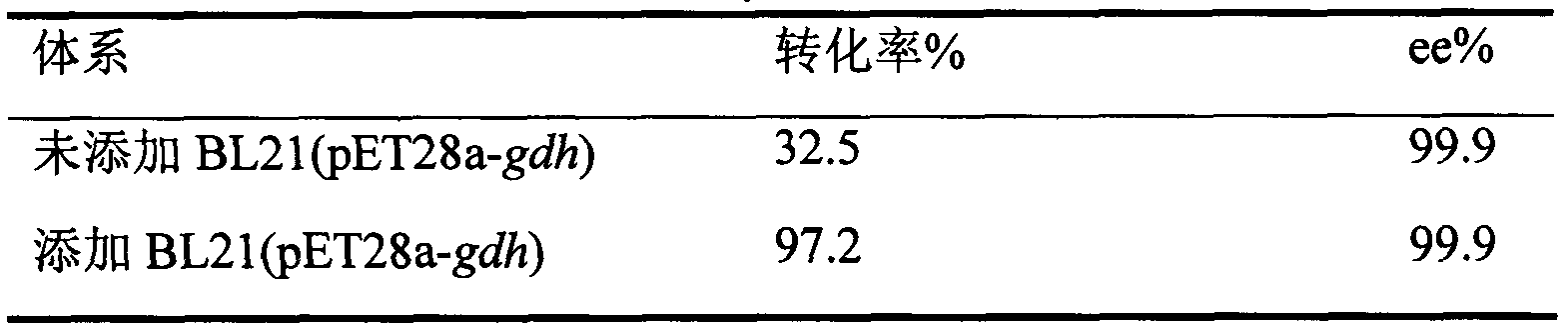
[0023] Get the thallus of Example 2 and wash twice with potassium phosphate buffer (100mmol / L, pH7.0), fix and weigh 0.5g (wet weight) of recombinant Escherichia coli BL21 (pET28a-cbr) thalline, add 2 times the amount The specific E. coli BL21 (pET28a-gdh) cells were suspended in 10 ml of pH 7.0 potassium phosphate buffer (containing 500 μl of dimethyldimethylamine). Add 0.1M glucose, 10g / L 2-bromo-4-nitroacetophenone, NAD(P)H0.5mmol / L, 37°C, 200rpm, the reaction is over for 12h, and the product (R)2-bromo-1-(4 The productive rate of -nitrophenyl)ethanol is 9.72g / L, and its productive rate is determined to be 97.2%, and the optical purity ee% is 99.9%.
[0024] After the water / organic solvent two-way system reaction was completed, 5ml of ethyl acetate was added, shaken vigorously for 5min, and left to separate the organic layer and the aqueous layer. Aspirate the upper ethyl acetate phase, add an appropriate amount of an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com