Ruthenium complex as well as preparation method and application thereof
A technology of ruthenium complexes and aromatic rings is applied in the field of ruthenium pyridine complexes with substituents and their preparation, which can solve the problems of myelosuppression drug resistance, toxicity and the like, and achieve the effects of increasing reaction temperature, low toxicity and speeding up reaction speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
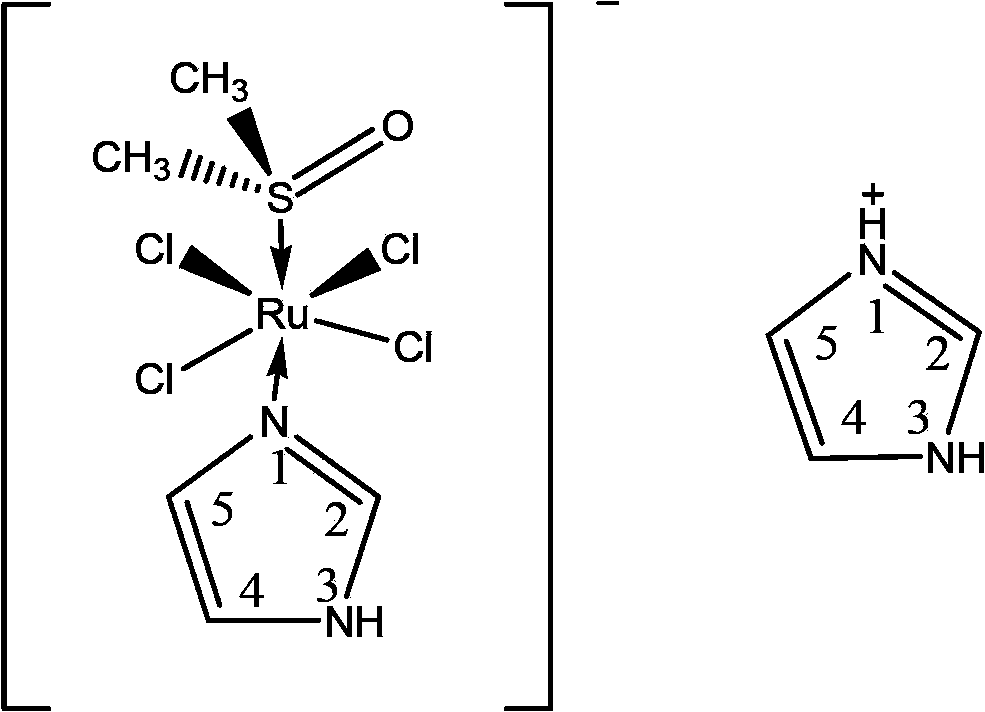
[0054] Example 1 [H(3-BrPy)][trans-RuCl 4 (DMSO)(3-BrPy)] Preparation (Compound 1, L=3-BrPy, 3-Bromopyridine)
[0055] Weigh the starting material trans-[RuCl 4 (DMSO) 2 ][(DMSO) 2 H] 0.10g (0.18mmol) was ground into a fine powder, dissolved in 8ml of acetone, and 0.07ml (0.72mmol) of 3-bromopyridine was added. The reaction was carried out ultrasonically for 3 hours, the orange powder produced was filtered and washed with acetone and ether, and the silica gel was dried overnight. Obtained orange powder: 0.10 g. Yield: 87%.
[0056] The physical and chemical properties of the obtained complex (compound 1) are described as follows: Morphology: orange needle-like crystallites. Molecular weight: 638.01. Melting point: 181-183°C. Molecular formula: C 12 H 15 Cl 4 Br 2 N 2 ORuS. Elemental analysis calculated value: C 22.59, H 2.37, N 4.39%; elemental analysis determined value: C 22.93, H 2.29, N 4.42%. UV / Vis(25℃,H 2 O)λmax, nm(ε,L·mol -1 ·Cm -1 ): 293(3280), 396(3980), 462(480). ...
Embodiment 2
[0057] Example 2 [H(4-BrPy)][trans-RuCl 4 (DMSO)(4-BrPy)] Preparation (Compound 2, L=4-BrPy, 4-Bromopyridine)
[0058] 4-Bromopyridine is unstable and must be converted to 4-bromopyridine after treatment with 4-bromopyridine hydrochloride.
[0059] Transformation process: Dissolve 1 g of 4-bromopyridine hydrochloride in 5 ml of water. Use 0.5mol·L -1 Adjust the pH value of NaOH solution to make it greater than 6. Extract with 3ml of chloroform and take out the chloroform layer (lower layer). Then repeat the extraction with 3ml chloroform respectively, combine the three extracts, and use anhydrous Na 2 SO 4 Dehydrate and reserve (store in refrigerator).
[0060] Weigh the starting material trans-[RuCl 4 (DMSO) 2 ][(DMSO) 2 H] 0.11g (0.20mmol), dissolved in 8ml of acetone, and 2ml of the above stock solution was added. After 3 hours of ultrasonic reaction, an orange solid powder was finally generated. Filter and wash with acetone and ether. The silica gel was dried overnight. Obt...
Embodiment 3
[0062] Example 3 [H(3-ClPy)][trans-RuCl 4 (DMSO)(3-ClPy)] Preparation (Compound 3, L=3-ClPy, 3-chloropyridine)
[0063] The preparation method is the same as in Example 1. Yield: 71.37%.
[0064] The physical and chemical properties are described as follows: Morphology: orange-yellow crystallites; molecular formula: C 12 H 15 Cl 6 N 2 ORuS; molecular weight: 549.11; elemental analysis calculated value: C 26.25, H 2.75, N 5.10%; measured value: C 26.25, H 2.87, N 4.71%. UV / Vis(25℃,H 2 O)λmax, nm(ε,L·mol -1 ·Cm -1 ): 290(3480), 396(4000), 462(490). Infrared spectrum characteristic peak (cm -1 ): 3089, 3050, 2916, 1516 (vs), 1465, 1452, 1421, 1073, 1022, 430 (s). 1 H-NMR spectrum (DMSO-d 6 ) δ: 8.76ppm, 8.62ppm, 8.11ppm, 7.60ppm, -1.96ppm, -12.85ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com