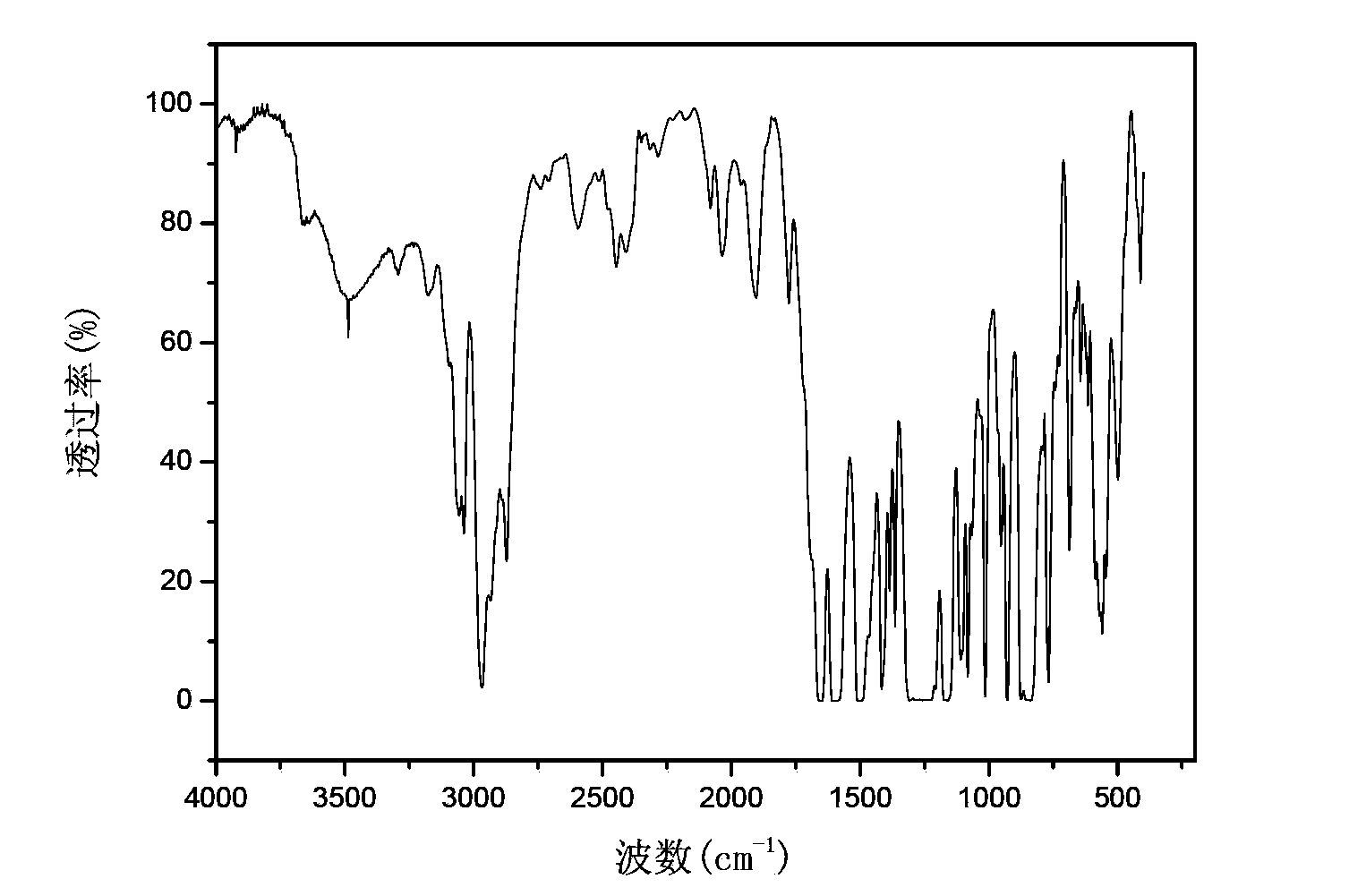
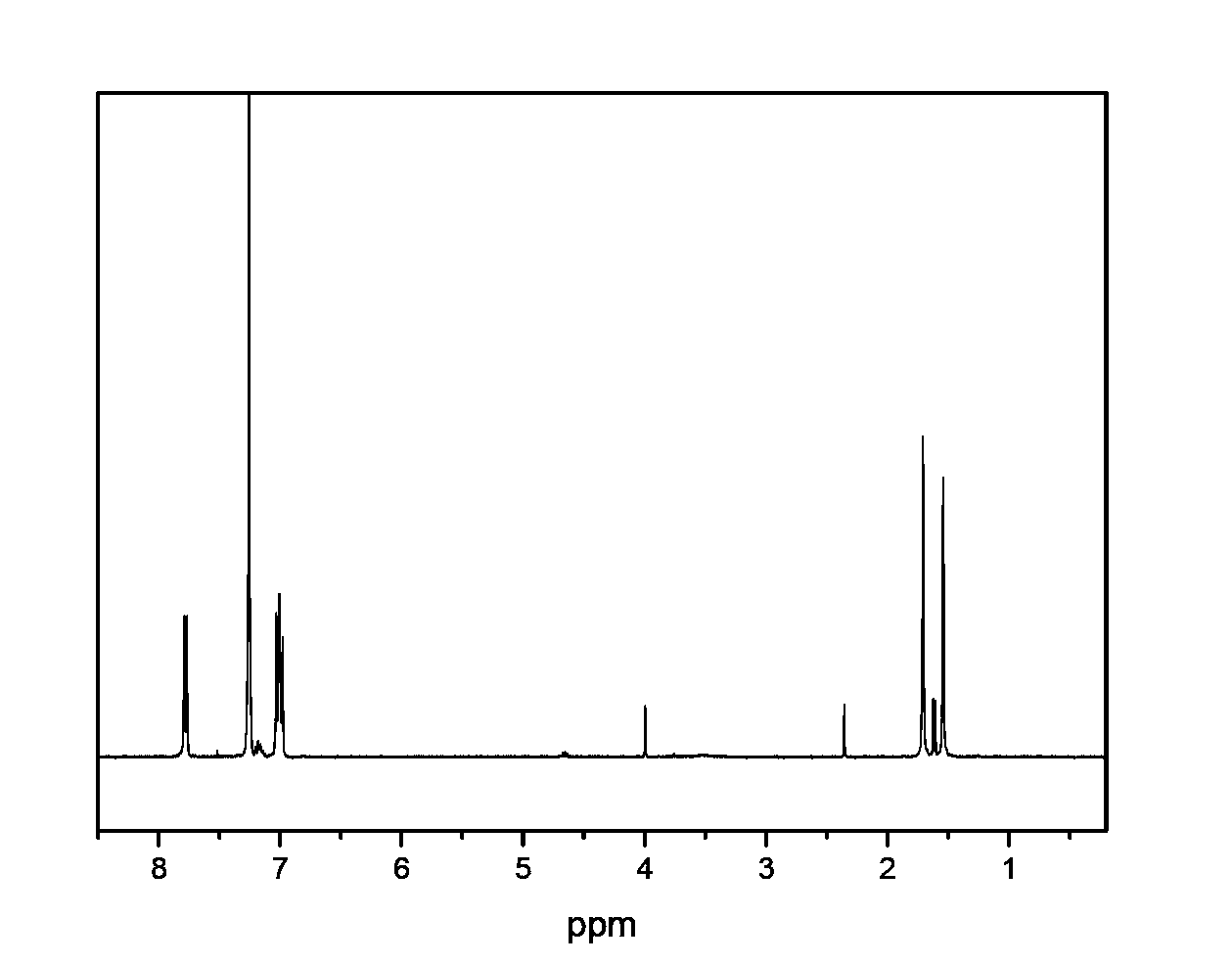
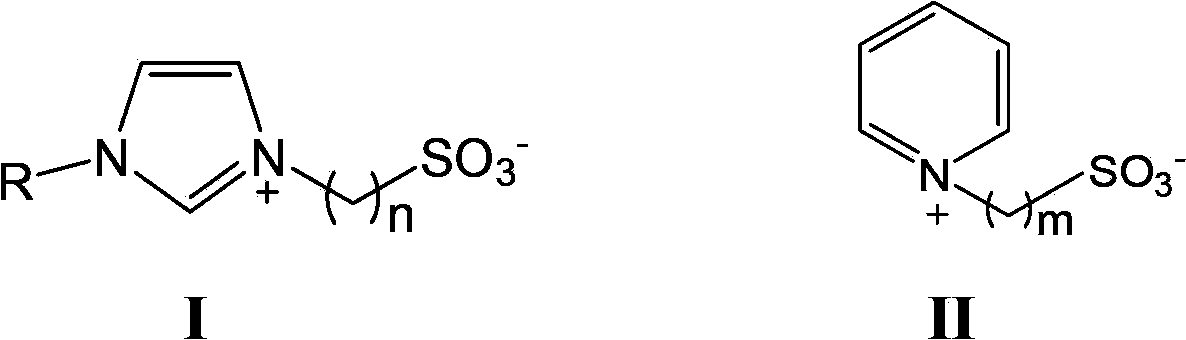Method of preparing polyaryletherketone
A poly(aryl ether ketone), polycondensation reaction technology, used in the production of bulk chemicals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1 : Synthesis of bisphenol A type polyaryletherketone a) in ionic liquid of group B) to form bisphenol A potassium salt
[0115] 0.639g (0.0028mol) of bisphenol A, 0.646g (0.0028mol) of 4,4'-difluorobenzophenone and ionic liquid [1-isopropyl-3-methylimidazole Hexafluorophosphate (i-pmim PF 6 )] 2.60g was added to a 100mL four-necked round-bottomed flask equipped with a thermometer, a stirrer and a condenser connected to a Dean-Stark water separator, vacuumed and vented with nitrogen for 3 times, and heated to 100°C in 15 minutes under a nitrogen atmosphere. ℃, the system is a homogeneous solution, start mechanical stirring, and then heat up to 140 ℃ after 10 minutes, then immediately add 0.42g (0.0030mol) of potassium carbonate and 5mL of toluene at one time, and then heat to 150 ℃ after 5 minutes, potassium carbonate is rapidly dispersed In the solvent, a white suspension is formed, and after reaching 150°C, bring water at this temperature for 0.5h, the ref...
Embodiment 2
[0118] Repeat the method described in Example 1, the difference is: adopt 1-isopropyl-3-methylimidazole Bromide salt (i-pmim Br) replaces 1-isopropyl-3-methylimidazole Hexafluorophosphate (i-pmim PF 6 ). After the white solid obtained after the polymerization was purified and dried, the yield of the crude polyaryletherketone was measured to be 91.1%. The crude product had a weight-average molecular weight of 17,300 and a polydispersity coefficient of 2.26.
Embodiment 3
[0120] Repeat the method described in Example 1, the difference is: adopt 1-isopropyl-3-methylimidazole Tetrafluoroborate (i-pmim BF 4 ) to replace 1-isopropyl-3-methylimidazole Hexafluorophosphate (i-pmim PF 6 ). After the white solid obtained after the polymerization was purified and dried, the yield of the crude polyaryletherketone was measured to be 89.7%. The crude product had a weight-average molecular weight of 12,000 and a polydispersity coefficient of 1.80.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com