Preparation method of PEG-PLA/PLA composite drug loaded nanometer microballoon
A PEG-PLA, drug-loaded nanotechnology, applied in the field of drug release carrier preparation, can solve the problems of large particle size, uneven particle size, cumbersome process, etc., and achieve simple preparation method, short reaction time, and improved solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
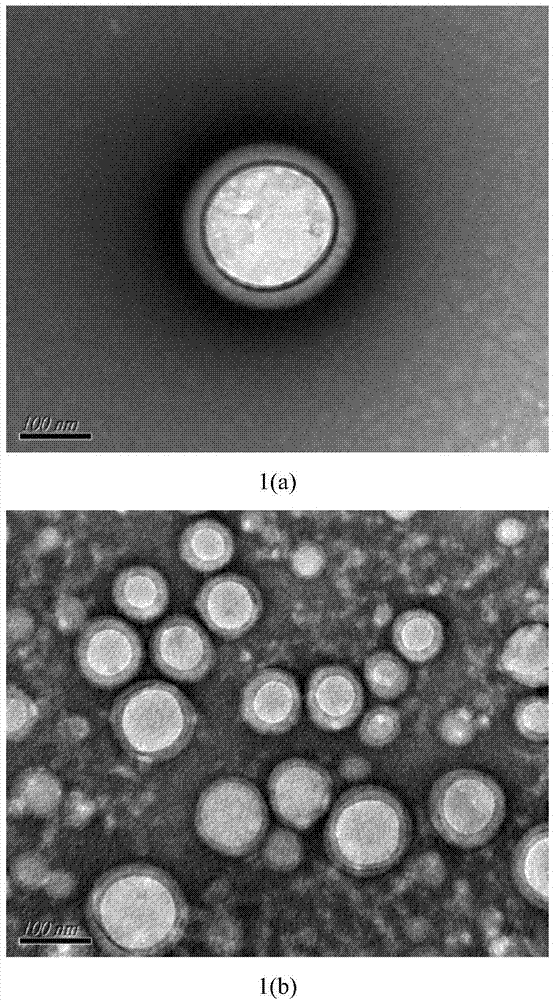
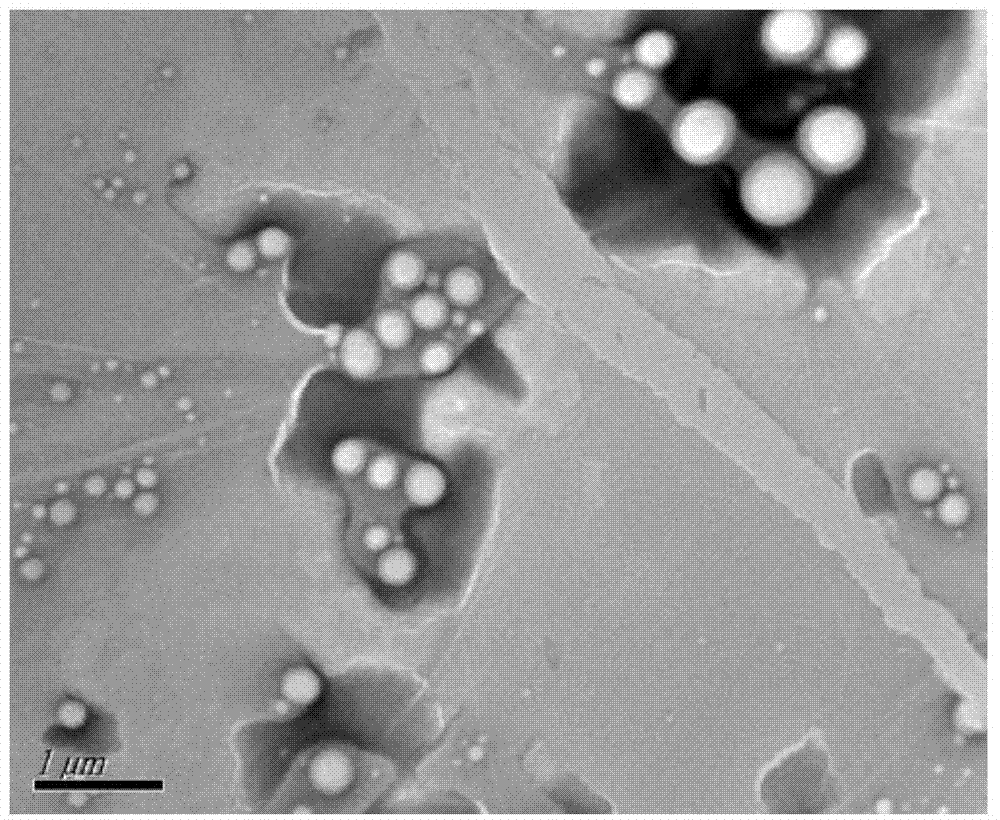
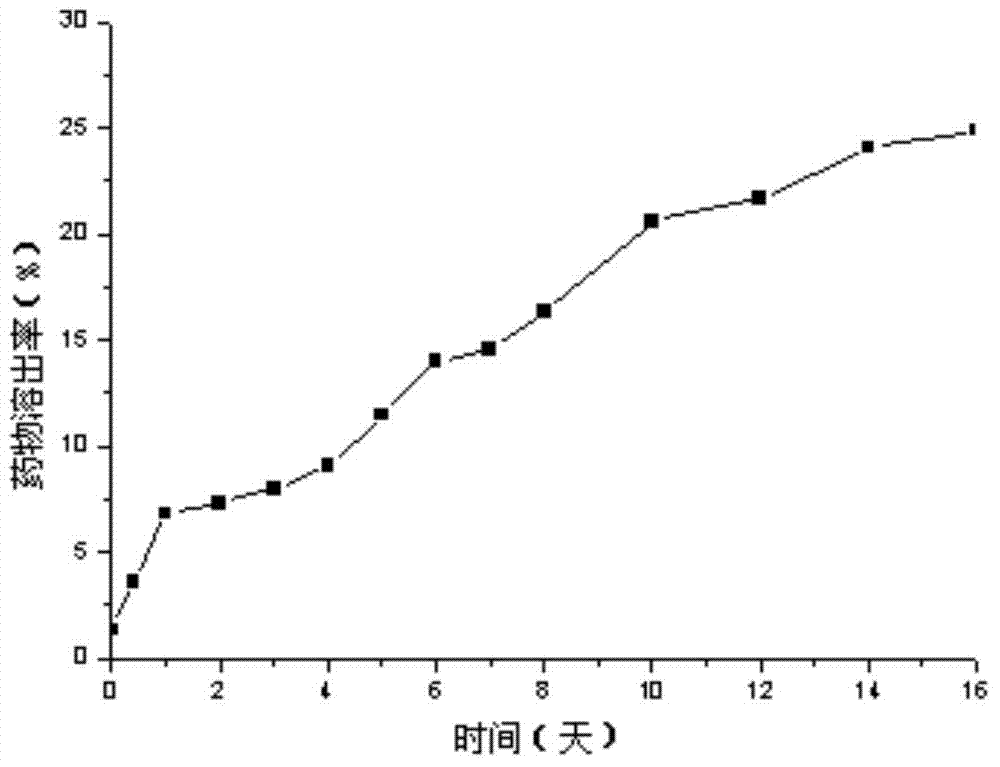
[0026] Weigh 200mg PEG-PLA (weight average molecular weight 8500), 100mg PLA (weight average molecular weight 30000), 30mg paclitaxel, add 2mL dichloromethane, 100w ultrasound for 1min to completely dissolve the carrier material and drug, then add 20mL deionized water to it , continue ultrasonication at 200w for 3min in an ice-water bath, then rise to room temperature, stir mechanically at 300rpm for 8h to completely volatilize the organic solvent, and finally freeze and centrifuge the drug-loaded microsphere emulsion at 15000rpm at 4°C for 15min at high speed, and freeze-dry the obtained solid to remove water and place it in Store in a sealed container at 4°C. The prepared paclitaxel-loaded PEG-PLA / PLA composite nanospheres showed a regular "core-shell" structure under TEM, with an average particle size of 300nm and an encapsulation efficiency of 80%.
Embodiment 2
[0028] Weigh 200mg PEG-PLA (weight average molecular weight 9800), 100mg PLA (weight average molecular weight 30000), 20mg paclitaxel, add 2mL dichloromethane, 300w ultrasonic 2min to completely dissolve the carrier material and drug, then add 10mL deionized water to it , continue ultrasonication at 200w for 10min in an ice-water bath, then rise to room temperature, ultrasonication at 200w for 1h to completely volatilize the organic solvent, and finally refrigerate and centrifuge the drug-loaded microsphere emulsion at 15000rpm at 4°C for 10min at high speed, and freeze-dry the obtained solid to remove water and place it in 4 ℃ sealed storage. The prepared paclitaxel-loaded PEG-PLA / PLA composite nanospheres showed a regular "core-shell" structure under TEM, with an average particle size of 250nm and an encapsulation efficiency of 75%.
Embodiment 3
[0030] Weigh 300mg PEG-PLA (weight average molecular weight 9800), 100mg PLA (weight average molecular weight 30000), 30mg paclitaxel, add 2mL dichloromethane, 500w ultrasound for 1min to completely dissolve the carrier material and drug, then add 30mL deionized water to it , continue ultrasonication at 300w for 15min in an ice-water bath, then rise to room temperature, mechanically stir at 900rpm for 20h to completely volatilize the organic solvent, and finally freeze and centrifuge the drug-loaded microsphere emulsion at 18000rpm at 4°C for 10min at high speed, and freeze-dry the obtained solid to remove water and place it in Store in a sealed container at 4°C. The prepared paclitaxel-loaded PEG-PLA / PLA composite nanospheres showed a regular "core-shell" structure under TEM, with an average particle size of 200nm and an encapsulation efficiency of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com