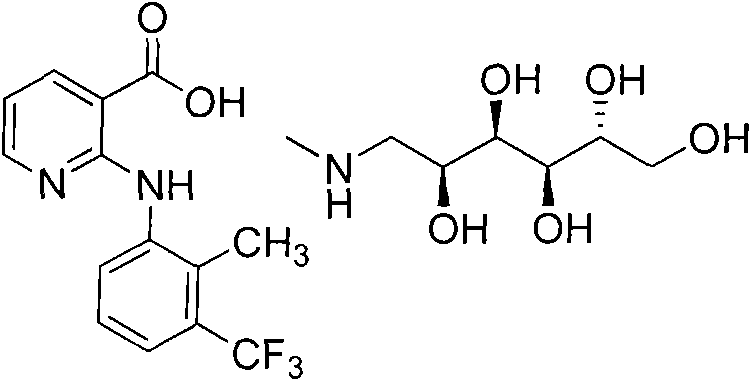
Method for synthesizing flunixin meglumine
A technology of flunixin meglumine and a synthesis method, applied in the field of veterinary drug synthesis, can solve the problems of complicated treatment, high reaction temperature, low yield and the like, and achieves the effects of convenient and simple operation, low equipment requirements, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of flunixin:
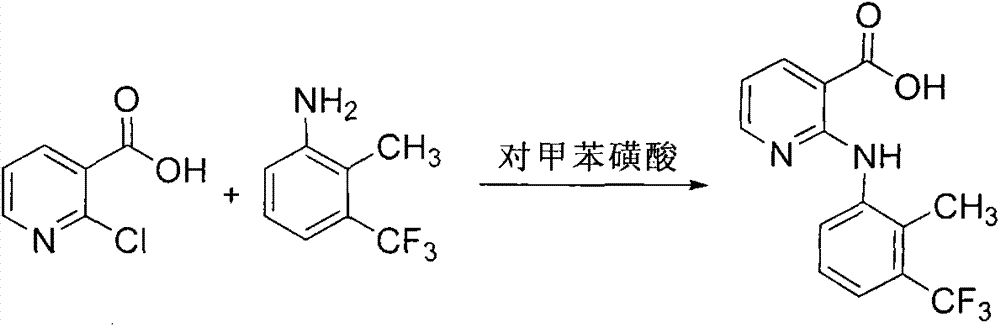
[0022] (1) Add 500g of purified water, 55.1g of 2-chloronicotinic acid, 122.6g of 2-methyl-3-trifluoromethylaniline, 0.6g of p-toluenesulfonic acid, and 0.28g of copper oxide into a 1000mL reaction flask equipped with a reflux tube , stirred and heated to reflux, and kept warm for 4 hours.
[0023] (2) The reaction solution is cooled to below 50°C, and 25% potassium hydroxide solution is added dropwise to the reaction solution to adjust the pH value to 10.0 to 11.0. After the adjustment, continue stirring and cool down to below 20°C, fully stir for 1 hour, and suction filter. The filter cake is mainly excess 2-methyl-3-trifluoromethylaniline, which can be recycled for further use.
[0024] (3) Add 30% sulfuric acid dropwise to the filtrate to adjust the pH value to 5.0-6.0, fully stir and crystallize for 1 hour, filter with suction, stir and wash the filter cake with 300 g of purified water for 30 minutes, filter, and dry. 94.5 g of the prod...
Embodiment 2
[0029] Preparation of Flunixin:
[0030] (1) Add 500g of purified water, 55.1g of 2-chloronicotinic acid, 122.6g of 2-methyl-3-trifluoromethylaniline, 3.0g of p-toluenesulfonic acid, and 1.4g of copper oxide into a 1000mL reaction flask equipped with a reflux tube , stirred and heated to reflux, and kept for 2.5 hours.
[0031] (2) The reaction solution is cooled to below 50°C, and 25% potassium hydroxide solution is added dropwise to the reaction solution to adjust the pH value to 10.0 to 11.0. After the adjustment, continue stirring and cool down to below 20°C, fully stir for 1 hour, and suction filter. The filter cake is mainly excess 2-methyl-3-trifluoromethylaniline, which can be recycled for further use.
[0032] (3) Add 30% sulfuric acid dropwise to the filtrate to adjust the pH value to 5.0-6.0, fully stir and crystallize for 1 hour, filter with suction, stir and wash the filter cake with 300 g of purified water for 30 minutes, filter, and dry. 98.5 g of the product ...
Embodiment 3
[0037] (1) Add 1000g purified water, 110.2g 2-chloronicotinic acid, 245.2g 2-methyl-3-trifluoromethylaniline, 12.0g p-toluenesulfonic acid, 5.6g copper oxide to a 2000mL reaction flask equipped with a reflux tube , stirred and heated to reflux, and kept warm for 2 hours.
[0038] (2) The reaction solution is cooled to below 50°C, and 25% potassium hydroxide solution is added dropwise to the reaction solution to adjust the pH value to 10.0 to 11.0. After the adjustment, continue stirring and cool down to below 20°C, fully stir for 1 hour, and suction filter. The filter cake is mainly excess 2-methyl-3-trifluoromethylaniline, which can be recycled for further use.
[0039] (3) Add 30% sulfuric acid dropwise to the filtrate to adjust the pH value to 5.0-6.0, fully stir and crystallize for 1 hour, filter with suction, stir and wash the filter cake with 300 g of purified water for 30 minutes, filter, and dry. 196.4 g of the product was obtained, the yield was 94.5%, and the HPLC p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com