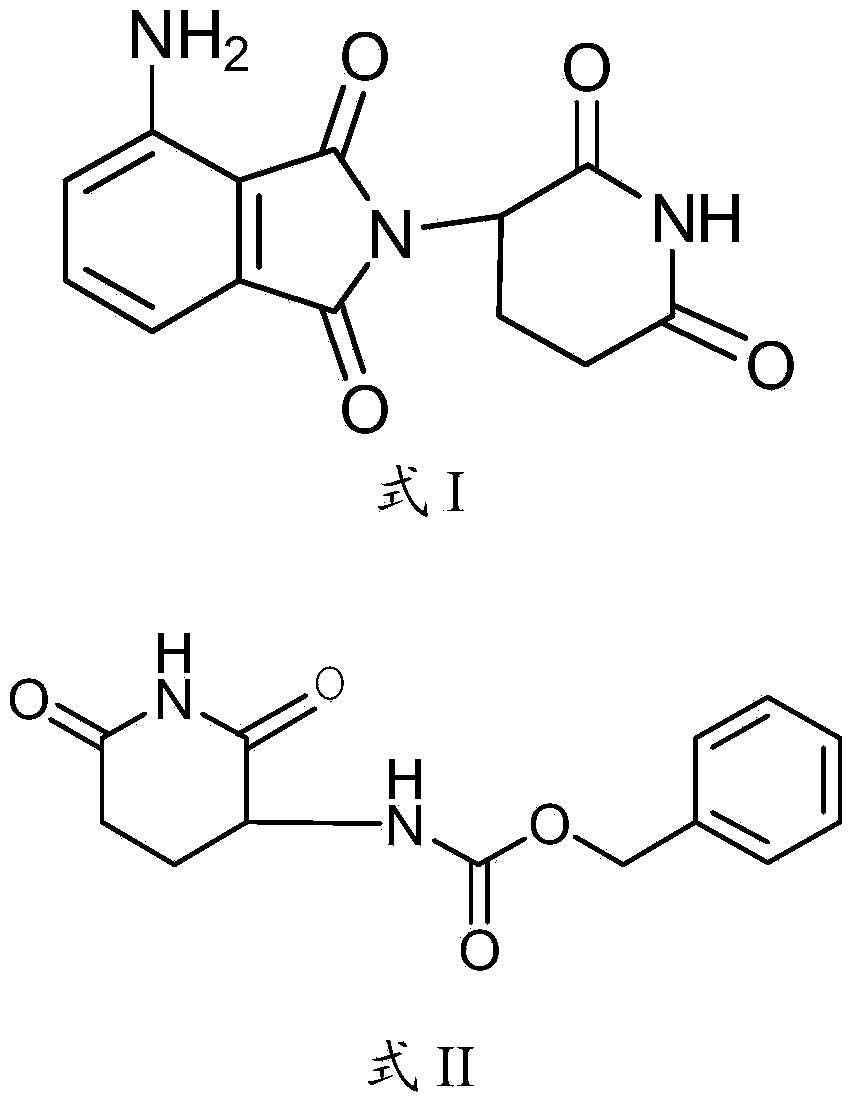
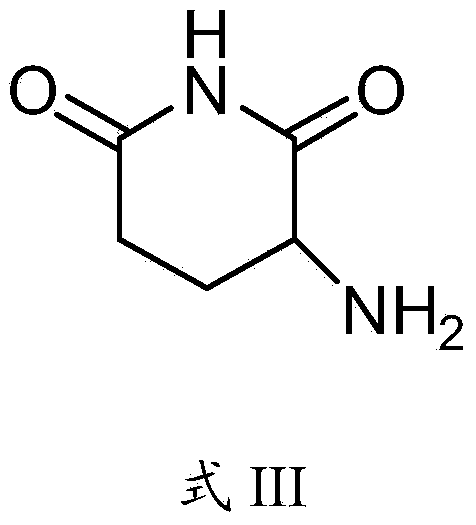
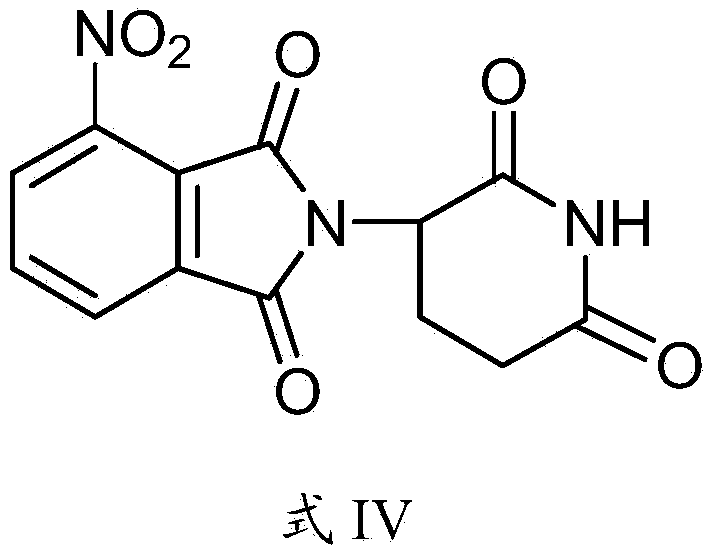
Preparation method of pomalyst pomalidomide
A technology of pomalidomide and glutarimide, applied in the field of pharmaceutical preparation, can solve the problems of long reaction period, difficult industrialization, pressurized hydrogenation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051]The invention discloses a preparation method of pomalidomide, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0052] The raw materials or reagents used in the preparation method of pomalidomide provided by the present invention can be purchased from the market.
Embodiment 1
[0054] The preparation of compound pomalidomide shown in embodiment 1 formula I
[0055] Add 56g of N-benzyloxycarbonyl-L-glutamine and 1000mL of acetone into the reaction flask, stir for 10 minutes and then add 49g of N,N'-carbonyldiimidazole. 57°C) until HPLC shows that the reaction of N-benzyloxycarbonyl-L-glutamine is complete, it takes 7 hours, after cooling, it is rotated to dryness at a bath temperature of 40°C, and 800 mL of saturated saline is added to the residue and stirred for 2 hours , suction filtration, washed with an appropriate amount of saturated saline, and dried to obtain 47.2g of white or off-white powder. After testing, the white or off-white powder is S-(-)-(2-phenoxycarboxamido) shown in formula II Glutarimide, purity 96.6%, yield 94.2%. The proton nuclear magnetic resonance spectrum data is: (CDCl 3 )δ=8.2 (s broad, 1H), 7.4 (s aromatic, 5H), 5.8 (d, 1H), 5.15 (s, 2H), 4.4 (dd, J=4.5, 3, 1H), 2.95-2.4 ( m, 3H), 1.86 (d, t, J=11.5, 6.5, 1H).
[0056...
Embodiment 2
[0063] The preparation of compound pomalidomide shown in embodiment 2 formula I
[0064] Add 56g of N-benzyloxycarbonyl-L-glutamine and 1000mL of dichloromethane into the reaction flask, stir for 10 minutes and then add 65g of dicyclohexylcarbodiimide. After the addition is complete, install a drying tube to stir and reflux the reaction (reflux temperature 41°C) until HPLC showed that the reaction of the raw material N-benzyloxycarbonyl-L-glutamine was complete, it took 4 hours, and after cooling, it was rotated to dryness at a bath temperature of 40°C, and 800 mL of saturated saline was added to the residue and stirred for 2 hour, suction filtration, appropriate amount of saturated salt water washing, drying, to obtain white or off-white powder 45.8g, after testing, white or off-white powder is S-(-)-(2-phenoxycarboxamide shown in formula II ) glutarimide, purity 96.2%, yield 91.4%. The proton nuclear magnetic resonance spectrum data is close to the result of embodiment 1. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com