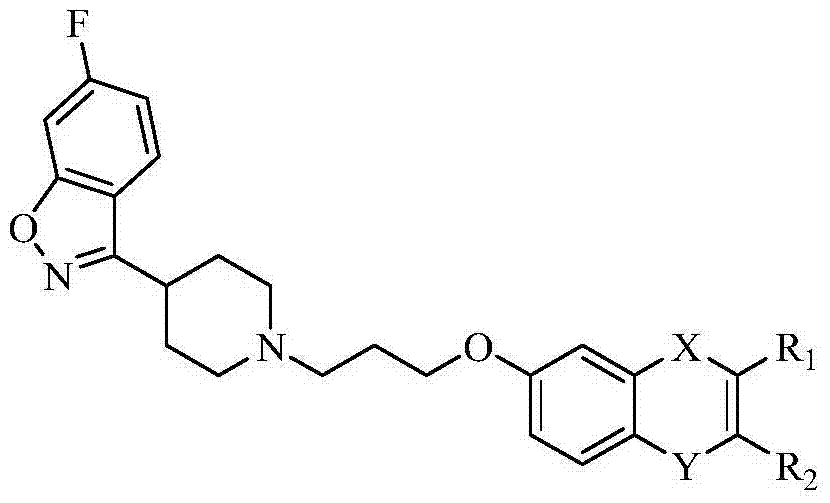
Benzisoxazole compound and application thereof
A compound, isoxazole technology, applied in the field of medicine, can solve problems affecting the DA function of the substantia nigra-striatum and tubercle-infundibulum system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: 3-{1-[3-(3,4-dihydro-2H-chromen-7-yloxy)propyl]pyridin-4-yl}-6-fluorobenzo[d]isox Preparation of azole
[0053] 2.82g (0.011mol) of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, 2.27g (0.01mol) of 7-(3-chloropropoxy)chroman mol), 5.29g (0.04mol) of anhydrous potassium carbonate and 0.17g (0.001mol) of potassium iodide were mixed in a 250mL round bottom flask, and 120mL of acetone was added as the reaction solvent, and heated to reflux for 24h. TLC monitoring showed that the reaction was complete and the product was single. Cool to room temperature, filter, wash the filter cake with acetone, combine the washing liquid with the filtrate, and concentrate to obtain a light yellow oil, which is separated by silica gel column chromatography, petroleum ether: ethyl acetate (v:v=1:2) to recover the reaction raw material 7-(3-Chloropropoxy)chroman. Ethyl acetate: triethylamine (v:v=100:1) to collect the target product. Concentration gave 2.25 g of wh...
Embodiment 2
[0054] Example 2: Preparation of 6-{3-[4-(6-fluorobenzo[d]isoxazol-3-yl)pyridin-1-yl]propoxy}chroman-4-one
[0055] According to the method of Example 1, a white solid was obtained with a yield of 54.5%, mp.135-136°C. 1 H-NMR (CDCl 3 ,600MHz)δ(ppm):7.33(1H,d,J=3.0Hz),7.25(1H,s),7.10(1H,d,J=3.0Hz),7.08(1H,d,J=3.0Hz) ,6.92(1H,d,J=9.0Hz),4.50(2H,t,J 1 =J 2 =6.6Hz), 4.05(2H,t,J 1 =J 2 =6.0Hz), 2.80(4H,m), 2.20(6H,m); ESI-MS: m / z425([M+H] + ),446.9([M+Na] + );IR,ν(cm -1 ):2952,2824,2773,1675,1613,1519,1434,1346,1282,1234,1122,1037,957,849,786,709.
Embodiment 3
[0056] Example 3: Preparation of 7-{3-[4-(6-fluorobenzo[d]isoxazol-3-yl)pyridin-1-yl]propoxy}chroman-4-one
[0057] According to the method of Example 1, a white solid was obtained with a yield of 59.7%, mp.122-123°C. 1 H-NMR (CDCl 3 ,600MHz)δ(ppm):7.83(1H,d,J=9.0Hz),7.70(1H,s),7.24(1H,q,J 1 =8.4Hz,J 2 =2.4Hz),7.05(1H,m,J 1 =8.4Hz,J 2 =8.4Hz,J 3 =2.4Hz),6.59(1H,q,J 1 =8.4Hz,J 2 =2.4Hz),6.43(1H,d,J=2.4Hz),4.51(2H,t,J 1 =J 2 =6.6Hz), 4.09(2H,t,J 1 =J 2 =6.6Hz), 3.08(3H,s), 2.75(2H,t,J 1 =J 2 =6.6Hz), 2.57(2H,s), 2.21(2H,m), 2.07(4H,m), 2.04(2H,m); ESI-MS: m / z425([M+H] + ),446.9([M+Na] + );IR,ν(cm -1 ):2957,2917,2849,2759,1675,1612,1578,1518,1495,1441,1257,1233,1197,1169,1122,1059,957,907,830,620.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com