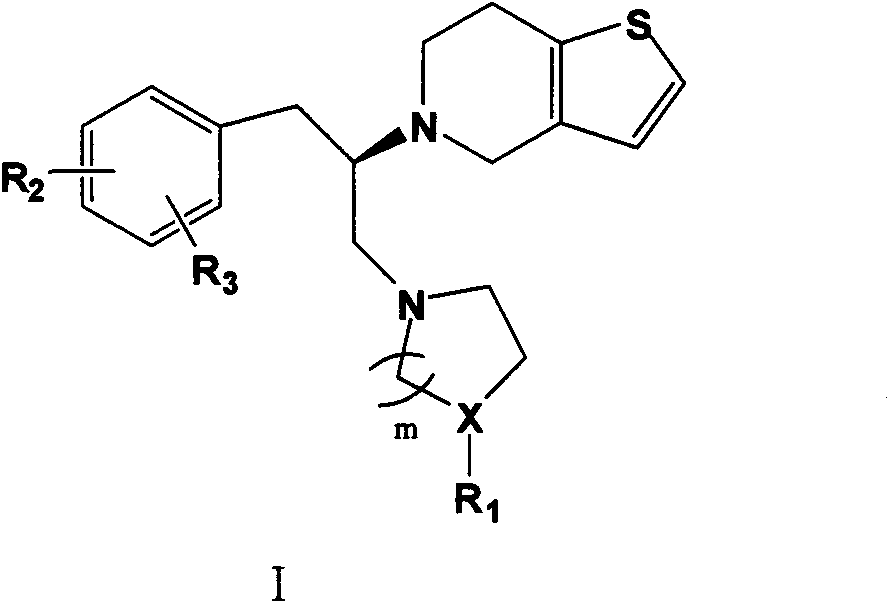
Thienopyridine compound and anti-platelet aggregative activity thereof
A compound and pyridine-based technology, applied in the field of medicine, can solve problems such as slow onset of action, large individual differences in platelet inhibition, and irreversible platelet effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (R)-5-[1-Benzyl-2-(4-methylpiperazinyl)]ethyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine oxalic acid Salt (I 1 )
[0050] Add (0.6g, 0.0021mol) intermediate 5((R)-5-(1-benzyl chloride ethyl)-4,5,6,7-tetrahydrothieno[3, 2-c] pyridine), 5ml acetonitrile, 0.43ml triethylamine, 0.03g KI and (1.15ml, 0.0105mol) N-methylpiperazine, stirred, and refluxed for 11 hours. Spin off the solvent, add 10ml ethyl acetate and 5ml water, stir for 10 minutes, let stand, separate the organic layer, extract the aqueous layer with ethyl acetate (10ml×3), combine the organic layers, wash with water, and dry with anhydrous MgSO4 , filtered, and spin-dried to obtain 0.64 g of a crude yellow oil, which was passed through a column to obtain 0.41 g of a pure light yellow oil. Yield 57.1%. IR (KBr, cm 1 )v: 3061, 3024, 2934, 2838, 2874, 1602, 1494, 1455, 1374, 1283, 1165, 1083, 1050, 1012, 747, 698; 1 H NMR (300MHz CDCl 3 )δ: 2.26 (3H, s, -NC H 3 ), 2.28-2.98 (16H, m, -PhC H 2 NC H 2 C H...
Embodiment 2
[0053] (R)-5-[1-benzyl-2-(4-ethylpiperazinyl)]ethyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Salt (I 2 )
[0054] Synthesize 0.38 g of the free base of I2 according to the synthesis method of I1. Yield 55.6%. IR (KBr, cm-1) v: 3061, 3024, 2927, 2808, 2360, 1602, 1452, 1379, 1275, 1165, 1013, 749, 698; 1 HNMR (300MHzCDCl 3 )δ: 1.05-1.09 (3H, t, -NCH 2 C H 3 ), 2.26-3.00 (18H, m, -PhC H 2 NC H 2 C H 2 -saifen-, -C H 2 N(C H 2 ) 4 NC H 2 CH 3 ), 3.17-3.19 (1H, m, -(CH 2 ) 2 C H -N-), 3.73-3.92 (2H, m, -N-CH2-saifen-), 6.73-6.75 (1H, d, J=6.0Hz, -C H =CH-S), 7.06-7.08 (1H, d, J=6.0Hz, -CH=C H -S-), 7.11-7.29 (5H, m, Ar H ); ESI-MS m / z: 370.3[M+H] + .
[0055] Get 0.2g free base and add oxalic acid to form a salt under the condition of isopropanol, get pale yellow solid (I 2 )0.29g, yield 98.3%, melting point: 168-169°C.
Embodiment 3
[0057] (R)-5-[1-benzyl-2-(4-n-propylpiperazinyl)]ethyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Oxalate (I 3 )
[0058] Add (0.54g, 0.0016mol) intermediate 6((R)-5-(1-benzylpiperazine ethyl)-4,5,6,7-tetrahydrothieno[3 , 2-c] pyridine), 5ml acetonitrile, 0.03g KI, 0.33ml triethylamine and (0.29ml, 0.032mol) n-propane bromide, stirred and refluxed for 12 hours. Suction filtration, spinning the filtrate to dryness, adding 10ml of dichloromethane to dissolve, washing with saturated NaCl, washing with water, drying with anhydrous NaSO4, filtering, spin-drying to obtain 0.33g of crude product of brown oil, which was purified by column to obtain 0.25g of light yellow oil. Yield 40.6%. IR (KBr, cm -1 )v: 3060, 3021, 2929, 1739, 1601, 1456, 1384, 1275, 1261, 1073, 749, 700; 1 H NMR (300MHz CDCl 3 )δ: 0.86-0.93 (3H, t, -N(CH 2 ) 2 C H 3 ), 1.45-1.55 (2H, m, -NCH 2 C H 2 CH 3 ), 2.26-2.98 (18H, m, -PhC H 2 NC H 2 C H 2 -saifen-, -C H 2 N(C H 2 ) 4 NC H 2 CH 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com