Butene lactone compound containing sulfonyl lactones as well as synthesis method and application thereof
A technology containing sulfonyl lactone and butenolide, which is applied in the directions of effective components of heterocyclic compounds, digestive system, organic chemistry, etc., can solve problems such as incompatibility, achieve good inhibitory effect, feasible synthesis route and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
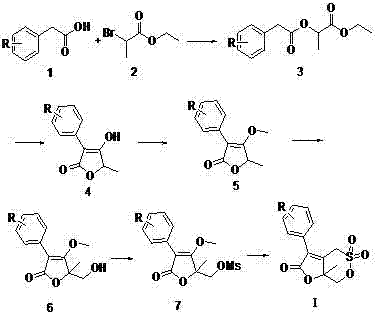
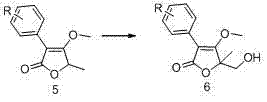
[0036] Embodiment 1: Preparation of compound Ia containing cyclic γ-lactone-δ-sultone structure
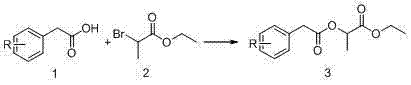
[0037]Step 1: Dissolve 10 g (58 mmol) 3,4-difluorophenylacetic acid in 100 mL tetrahydrofuran, add 15.78 g (87 mmol) ethyl α-bromopropionate, 8.79 g (87 mmol) triethyl The amine was heated to 60-70°C and reacted for 7-8 h, followed by TLC monitoring. After the reaction was completed, it was filtered with suction and concentrated to obtain a yellow oily compound 3a crude product.
[0038] Step 2: Dissolve the crude compound 3a in 60 mL DMF, add potassium tert-butoxide 9.74 g (87 mmol) in 6 times in an ice bath, after the addition is complete, continue to stir in the ice bath for 0.5 h, and then leave to stir at room temperature After 12 h of reaction, TLC monitored the completion of the reaction. To deal with the reaction, first dilute the system with 20 mL of water, then adjust the pH value of the system to 3-4 with dilute hydrochloric acid, and then add about 200 mL of water to ...
Embodiment 2
[0043] Embodiment 2: table 1 adopts the method described in embodiment 1 to prepare following compound
[0044] Compounds R Ia m,p -diF Ib o -F IC m -F ID p -F Ie H If o -Br Ig p -Br Ih p -CH 3 o II p -CF 3 Ij p -CH 3 Ik p -Cl
[0045] Ia
[0046] 1 H NMR (400 MHz, CDCl 3, TMS) δ 7.31 (d, J = 8.3 Hz, 3H), 4.69 (d, J = 13.8 Hz, 1H), 4.60 (d, J = 10.7 Hz, 1H), 4.42 (d, J = 10.7 Hz, 1H), 4.22 (d, J = 13.8 Hz, 1H). 13C NMR (101 MHz, CDCl 3 , TMS) δ 168.73, 151.73, 125.50, 125.46, 118.52, 118.42, 118.34, 118.24, 78.76, 77.23, 76.87, 49.42, 20.79. IR 1764.28 C=O , 1173.69 SO2 (s), 1369.27 SO2 (as).
[0047] Ib
[0048] 1 H NMR (400 MHz, CDCl 3, TMS) δ 7.63 (d, J = 1.6 Hz, 1H), 7.51 (ddd, J = 15.5, 5.4, 1.7 Hz, 1H), 7.33 (s, 1H), 7.23 (s, 1H), 4.59 (d, J = 10.8 Hz, 1H), 4.47 (s, 1H), 4.45 (d, J = 2.6 Hz, 1H), 4.26 (d, J = 14.2 Hz,...
Embodiment 3
[0067]Embodiment 3: In vitro HCV activity screening experiment of the compound of the present invention
[0068] 1 Material:
[0069] Bovine kidney cells (MDBK) cultured in RMPI-1640 medium containing 10% fetal bovine serum (FBS); bovine viral diarrhea prepared by three times of plaque purification in prepared bulk monolayer bovine kidney cells (MDBK) Pathogenic strain AV-69 of virus (BVDV).
[0070] 2 methods:
[0071] a Anti-HCV activity assay
[0072] Bovine kidney cells (MDBK) were seeded in 96-well plates after counting, 2×10 per well 4 Cells were cultured in a 37°C, 5% carbon dioxide incubator for 24 hours, and the supernatant was discarded, and 100 uL of bovine viral diarrhea virus (100TCID50) was added to each well. Two hours later, the culture medium was removed, and each well was washed twice with 100 uL of phosphate-buffered saline (PBS), and the drug solution prepared with the culture medium at a concentration of 0.01 μM-1 μM was immediately added. The culture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com