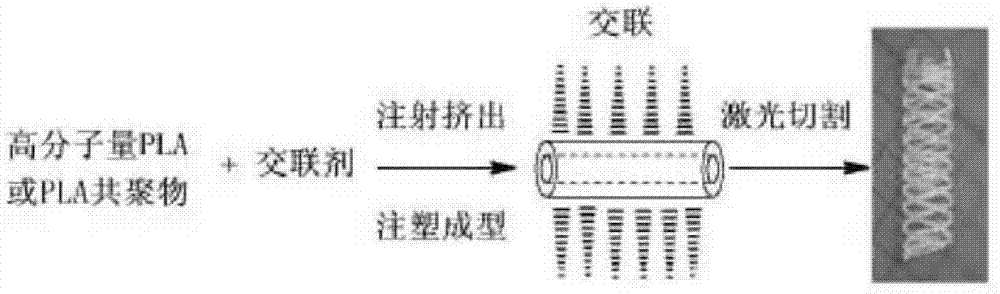
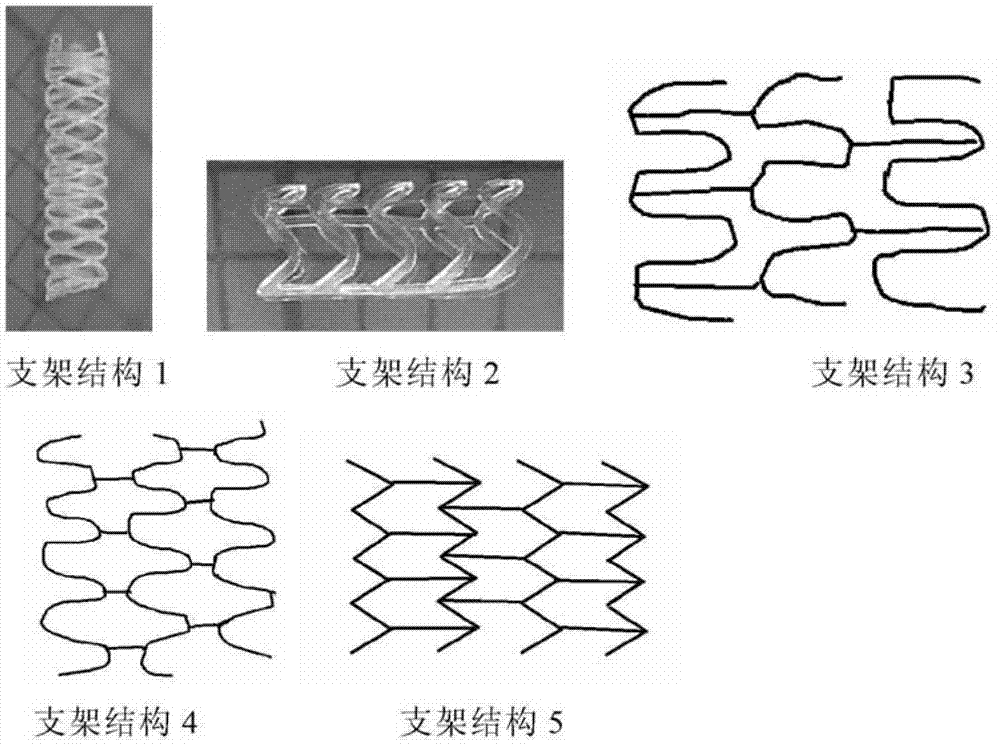
Modified polylactic acid degradable stent and preparation method thereof
A technology of polylactic acid and polylactic acid copolymer, which is applied in the field of medical devices, can solve problems such as uneven wall thickness of pipes, decrease in radial support force, and rapid decrease in radial support force, so as to reduce mechanical relaxation behavior and increase diameter. The effect of supporting force and enhancing the stability of the structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
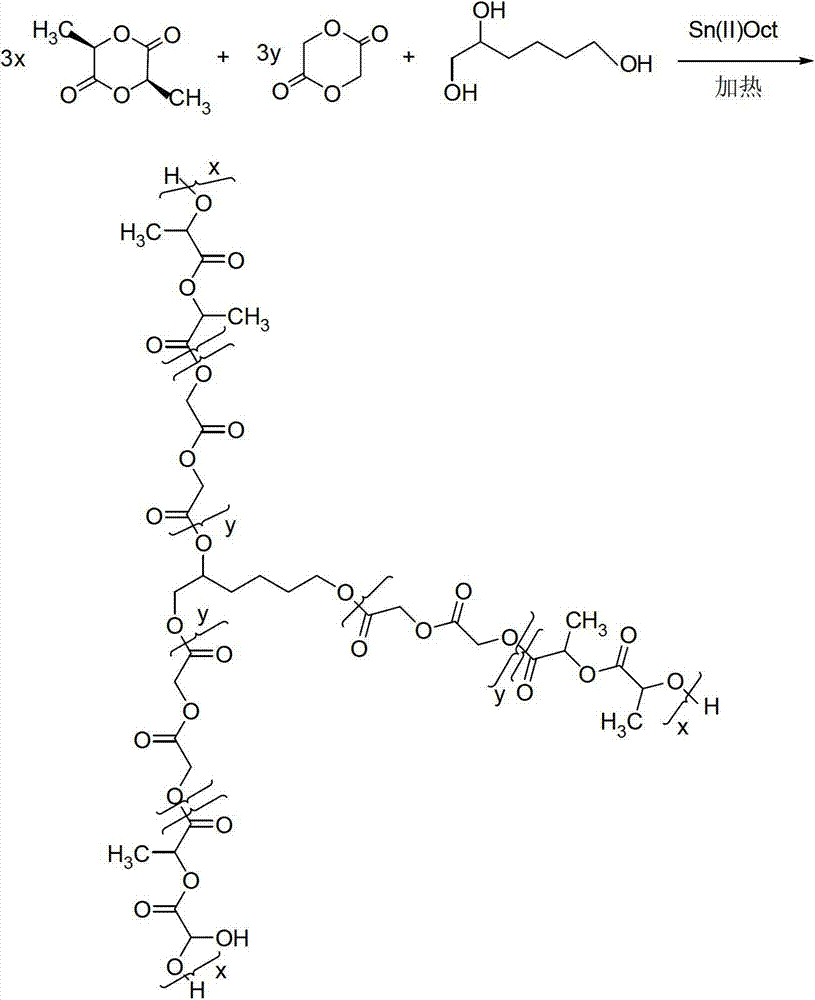
[0053] Example 1: Synthesis of 3-arm star crosslinkers
[0054] Before polymerization, the 3-liter glass reactor was vacuum-dried at 60°C for 1 hour, and 2000g L-lactide (L-lactide), 100g glycolide (glycolide) and 14g 1,2,6-hexyl The triol was added into the reactor and dried under vacuum at 60°C for 1 hour. Then add 2g of stannous octoate, increase the temperature to 140°C, and keep it at 140°C for 3 hours to obtain a star-shaped polylactic acid prepolymer with a number average molecular weight of 20,000 (see Reaction Formula 1 with three hydroxyl groups (n =3) degradable polymer).
[0055] Reaction 1: Formation of a star PLA prepolymer with 3 arms
[0056]
[0057] Among them, x=3-300, y=1-100.
[0058] For clarity, the above structure simplifies to:
[0059]
[0060] That is, a degradable polymer with three hydroxyl groups (n=3).
[0061] Using a similar method, prepolymers of polylactic acid or polylactic acid copolymers of different compositions can be synthesi...
Embodiment 2
[0066] Example 2: Synthesis of 2-arm linear crosslinkers
[0067] Before polymerization, the 3-liter glass reactor was vacuum-dried at 60°C for 1 hour. Under nitrogen protection, 2000g of L-lactide and 50g of Poly(THF) were added to the reactor, and vacuum-dried at 60°C for 1 hour. Then add 2g of stannous octoate, increase the temperature and react at 140°C for 3 hours to obtain a linear polylactic acid prepolymer with a number average molecular weight of 20,000. The molecular weight of the linear polylactic acid prepolymer is controlled by the relative content of the initiator and the monomer, and the number average molecular weight is controlled between 5,000 and 50,000. When the molecular weight of the star-shaped polylactic acid copolymer prepolymer meets the requirements of the experimental design, directly add isocyanoethyl methacrylate and 300ppm free radical inhibitor p-hydroxyanisole to form a cross-linkable linear polymer (See Equation 3).
[0068] Reaction 3: Synt...
Embodiment 3
[0071] Example 3: Synthesis of a 4-arm star crosslinker
[0072] Before polymerization, vacuum-dry the 3-liter glass reactor at 60°C for 1 hour, add 2000g of L-lactide, 100g of caprolactone (ε-caprolactone), and 60g (0.44mol) of pentaerythritol into the reactor. Vacuum dry at 60°C for 1 hour. Then add 2g of stannous octoate, increase the temperature to 125°C and keep it at 140°C for 3 hours to obtain a polylactic acid prepolymer with a number average molecular weight of 18,000 (see Reaction Formula 4 with four hydroxyl groups (n=4) degradable polymers).
[0073] The molecular weight of the prepolymer is controlled by the relative content of initiator and monomer, and the number average molecular weight is controlled between 5,000 and 50,000. When the molecular weight of the star-shaped polylactic acid prepolymer meets the requirements of the experimental design, directly add 72g (0.47mol) methacrylic anhydride and 0.6g free radical inhibitor (p-hydroxyanisole) to form a cros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| verticality | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com