Method and culture media for inducing osteogenic differentiation of induced pluripotent stem cell of mouse
A technology of differentiation medium and pluripotent stem cells, which is applied in the field of induction of mouse induced pluripotent stem cells to differentiate into bone, can solve the problems of unclear medium composition and low purity of differentiated cells, and achieve broad application prospects and practical value. High differentiation rate and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
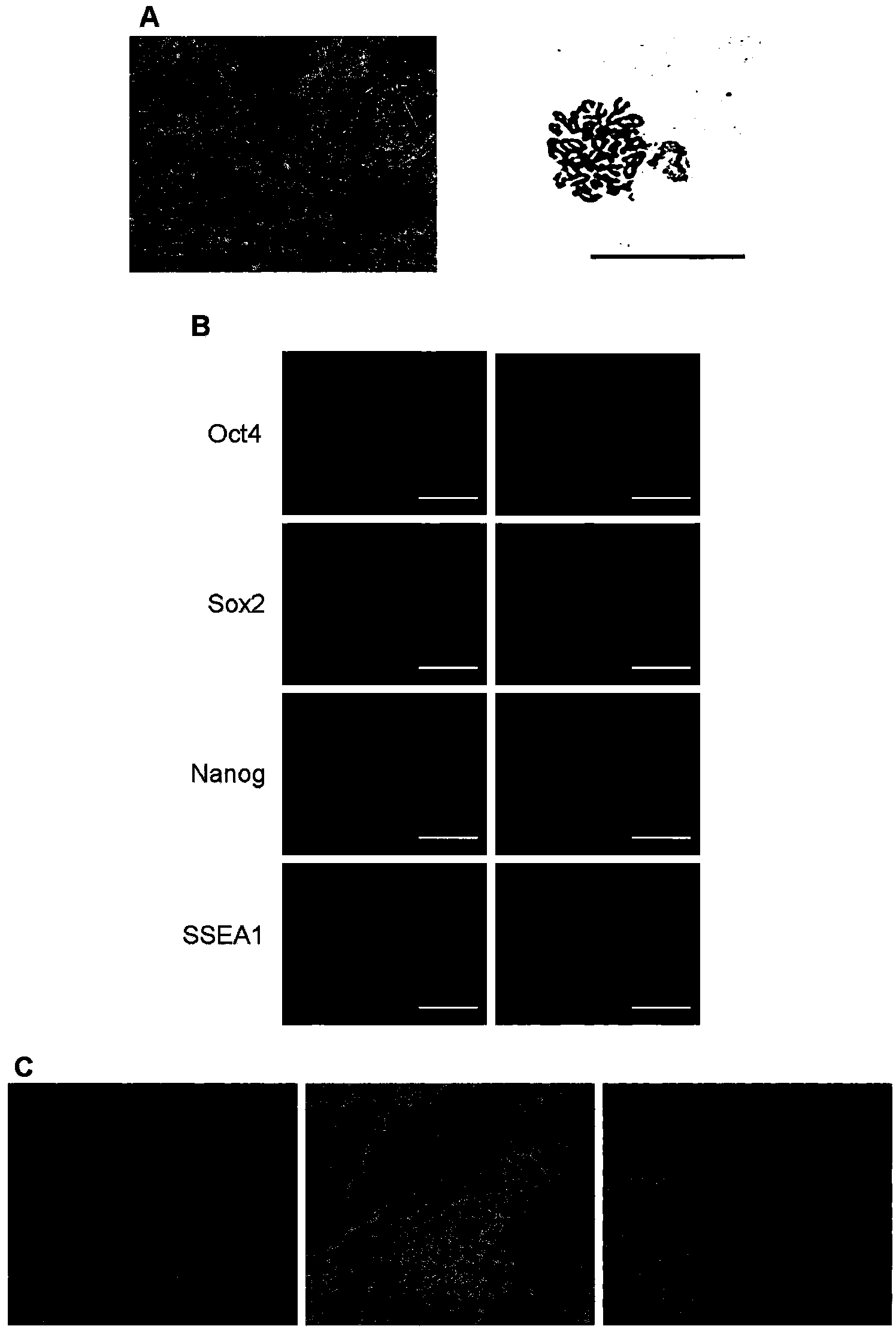
[0035] Example 1 Culture and Identification of Mouse iPS Cells
[0036] Obtaining and maintaining mouse iPS cell lines with good pluripotency and differentiation ability is the basis for cell-directed differentiation in the later stage. This example is a description of the operation process of culturing and identifying mouse iPS cells used in the present invention.
[0037] 1. Culture of mouse iPS cells
[0038] The mouse iPS cell line p14-2-2 used in this example was provided by the Chinese Academy of Sciences Stem Cell Bank.
[0039] Mitomycin C working solution: Dissolve 2mg of Mitomycin C in 200mL high-glucose DMEM containing 10% fetal bovine serum, and filter to sterilize.
[0040] 0.1% gelatin: Dissolve 0.1g gelatin powder in 100mL double distilled water and autoclave.
[0041] Fibroblast culture medium: high-glucose DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin.
[0042] LN culture medium: containing 48%DMEM / F12, 48%Neurobasal, 1000U / mL LIF, ...
Embodiment 2
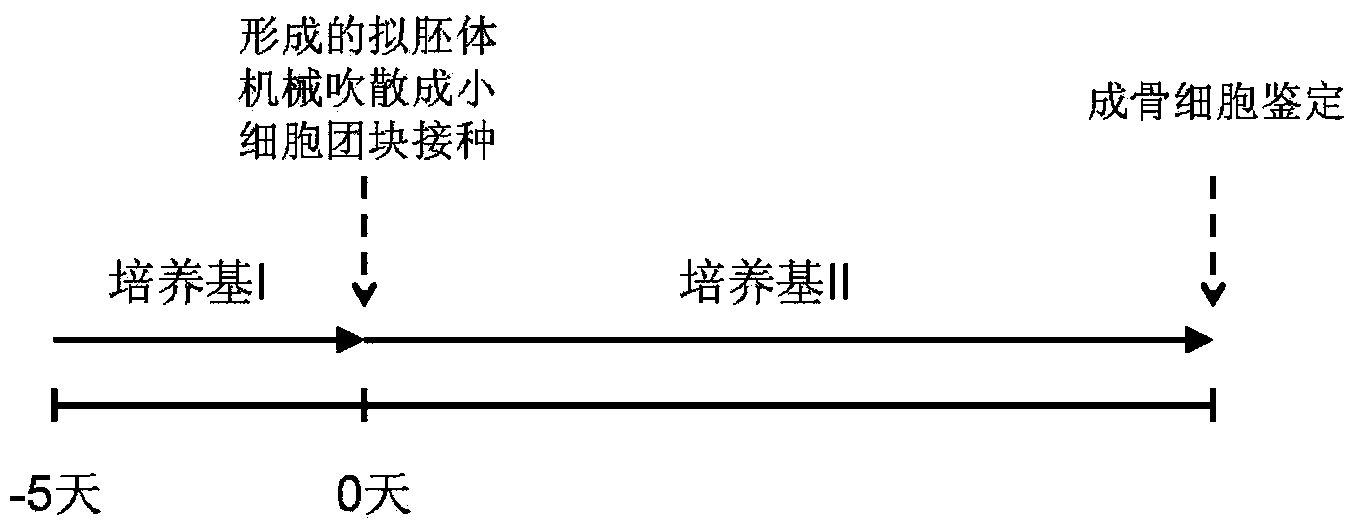
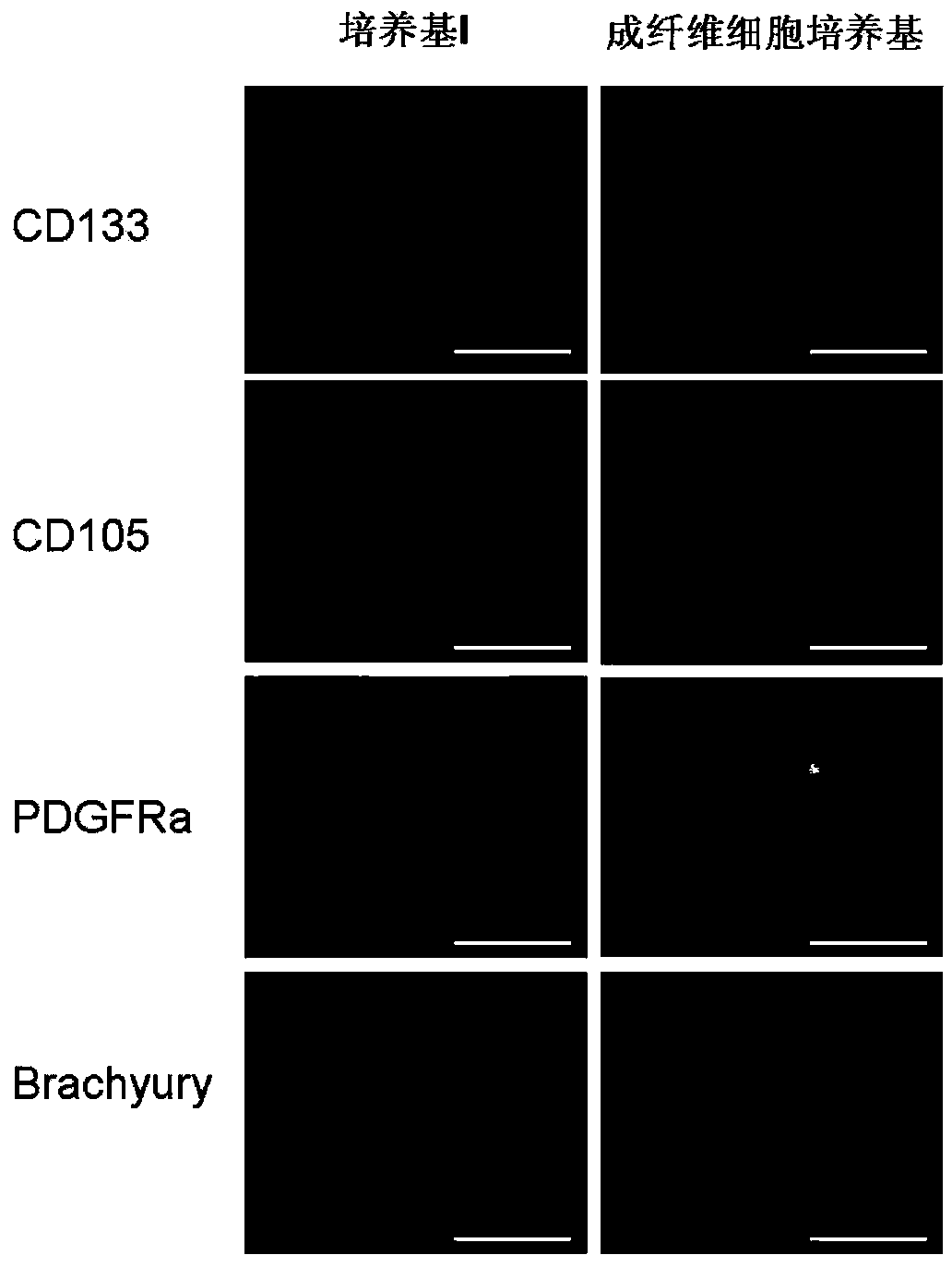
[0055] Example 2 Osteotropic differentiation of mouse iPS cells
[0056] In the present invention, the osteotropic differentiation of mouse iPS cells is accomplished through two steps: the first step is to obtain embryoid bodies enriched in mesenchymal cells under appropriate culture conditions; and the second step is to induce differentiation Obtain osteoblasts. Operation process see figure 1 .
[0057] 1. Preparation of embryoid bodies
[0058] Differentiation medium I: add 5×10 to the fibroblast culture medium -8 mol / L all-trans retinoic acid and 10ng / mL bFGF, pH 7.0-7.4. Wherein, the fibroblast culture medium is high-sugar DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin.
[0059] The operation process of this step includes: referring to Example 1, removing the feeder layer cells and collecting the suspension cells by gelatin attachment method; adjusting the cell density to 2×10 5 cells / mL, inoculate cells into ultra-low adhesion culture dishes;...
Embodiment 3
[0066] Example 3 Identification of osteogenic differentiated cells
[0067] 1. Calcium deposition level detection
[0068] 1% Alizarin Red S: 0.1M tris-Hcl 100mL (pH8.3), add 0.1g Alizarin Red S, store at 4°C.
[0069] In this example, the Alizarin red staining method was used to detect the calcium deposition level of differentiated cells, and the steps included: induction of differentiation medium I for 5 days (step 1 of Example 2) and induction of differentiation medium II for 14 days (Example 2 Remove the culture medium from the cells in step 2), wash with PBS 3 times; fix the cells with 95% ethanol at room temperature for 10 minutes, wash 3 times with PBS, add 1% Alizarin Red S staining solution, incubate at 37°C for 30 minutes; wash with PBS Cells were washed 3 times, observed and photographed under an inverted microscope.
[0070] The staining result is as Figure 4 As shown (where, A is the control group; B is the osteoblast medium group; C is the differentiation mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com